Invited Symposium: What Can Genetic Models Tell Us About Attention-Deficit Hyperactivity Disorder (ADHD)?
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The catecholamine dopamine (DA) is an important regulator of many neural functions, including motor integration, neuroendocrine hormone release, cognition, emotive behaviors and reward (1). In mammals DA neurons are relatively few, when compared to the total number of brain neurons. They are mainly located in the ventral midbrain to form the retrorubral nucleus (A8), the substantia nigra (area A9) and the ventral tegmental area (area A10) (2). In rodents, neurons arising from the substantia nigra project to the striatum (corresponding to the caudate-putamen in primates) and receive innervation from multiple structures in the diencephalon and telencephalon. The ascending nigrostriatal pathway regulates motor control and its degeneration in humans is associated with Parkinson's disease, the syndrome described by the English neurologist in his “Essay on the shaking palsy” in 1817. Neurons from the ventral tegmental area project to the limbic system and cortex, and are involved in emotional and reward behavior and in motivation (1). Disturbances in this system have been associated with schizophrenia (although direct evidence is still elusive), addictive behavioral disorders and attention-deficit hyperactivity disorder (ADHD) (3, 4). DA also modulates interactions between prefrontal cortex and visual association areas, which are important in visual memory (5). The retrorubral A8 DA neurons projects to the substantia nigra and ventral tegmental area and seem involved in the coordination of the nigrostriatal and mesolimbic systems.
At the biochemical level, DA is synthesized from tyrosine by tyrosine hydroxylase (TH); its metabolism and function are summarized in Figure 1.
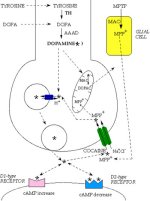 Click to enlarge
Fig. 1: Dopamine metabolism. Tyrosine is converted to L-3,4-dihydroxyphenylalanine (L-DOPA) by tyrosine hydroxylase (TH) and L-DOPA is converted to dopamine (DA, star) by aromatic L-amino acid decarboxylase (AAAD). DA can be further converted into noradrenaline by dopamine-beta-hydroxylase enzyme and noradrenaline into adrenaline by phenyl-ethanol-amine-N-methyl-transferase (pathways not shown in the figure). DA is transported into vesicles by the synaptic vesicle transporter VMAT2 (small blue ovals with arrows). When vesicles fuse with the presynaptic plasma membrane, DA is released into the synaptic cleft and interacts with postsynaptic D1-type or D2-type receptors (which modulate cAMP level). DA action at the synapse is terminated predominantly by re-uptake into the presynaptic terminal through the dopamine transporter DAT (large green ovals with arrows). DAT is blocked by cocaine and can transport the dopaminergic neurotoxin 1-methyl-4-phenylpyridinium, which derives from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), by the action of the glial monoamine oxidase, MAO. Once back into the DA terminals, the neurotransmitter can be repackaged into vesicles or catabolized by mitochondrial MAO into 3,4 dihydroxylphenylacetic acid (DOPAC).
Click to enlarge
Fig. 1: Dopamine metabolism. Tyrosine is converted to L-3,4-dihydroxyphenylalanine (L-DOPA) by tyrosine hydroxylase (TH) and L-DOPA is converted to dopamine (DA, star) by aromatic L-amino acid decarboxylase (AAAD). DA can be further converted into noradrenaline by dopamine-beta-hydroxylase enzyme and noradrenaline into adrenaline by phenyl-ethanol-amine-N-methyl-transferase (pathways not shown in the figure). DA is transported into vesicles by the synaptic vesicle transporter VMAT2 (small blue ovals with arrows). When vesicles fuse with the presynaptic plasma membrane, DA is released into the synaptic cleft and interacts with postsynaptic D1-type or D2-type receptors (which modulate cAMP level). DA action at the synapse is terminated predominantly by re-uptake into the presynaptic terminal through the dopamine transporter DAT (large green ovals with arrows). DAT is blocked by cocaine and can transport the dopaminergic neurotoxin 1-methyl-4-phenylpyridinium, which derives from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), by the action of the glial monoamine oxidase, MAO. Once back into the DA terminals, the neurotransmitter can be repackaged into vesicles or catabolized by mitochondrial MAO into 3,4 dihydroxylphenylacetic acid (DOPAC).
Upon release from the presynaptic terminal into the synaptic cleft, DA acts through D1R (D1 and D5) and D2R (D2, D3, D4) subfamilies of G-protein-coupled receptors (6). DA neurotransmission is terminated by uptake of the released messenger into the presynaptic DA fibers.
The physiological role and clinical relevance of dopaminergic neurons are well recognized. The mechanisms underlying their development have been the object of intense investigation and now we begin to understand many fundamental details (7).
The precise anatomical localization and functional differentiation of DA neurons in the mammalian brain is achieved through the action and gradient disposition of various diffusible factors. Data from tissue transplantation and explant culture studies and biochemical and genetic experiments have demonstrated that DA neurons develop at sites where the signals of two distinct molecules, Sonic hedgehog (SHH) and FGF8, intersect. These two extracellular inducers are both necessary and sufficient for the induction of DA neurons.
The latter and other still unidentified inductive molecules, are thought to activate cascades of other signaling molecules and transcription factors which lead to the final differentiation of DA neurons. Two transcription factors, Nurr 1 and the bicoid related Ptx3, expressed at crucial times in differentiating midbrain DA cells, have been recently identified. Absence of Nurr 1 in knock out mice leads to agenesis of midbrain DA neurons and the consequent lack of striatal dopamine innervation (8). Differently from Nurr 1, which is also expressed in other CNS regions, Ptx3 expression remains restricted to the mesencephalic DA system (9). In the absence of Nurr 1, neuroepithelial cells that should give rise to DA neurons adopt a normal ventral localization and express Ptx3. However, these late precursors fail to differentiate and undergo apoptosis (10).
In order to understand how DA neuronal circuit is formed during ontogeny and maintained in adult life, we have investigated:
-the ontogeny of DA neurons in vivo and in vitro;
-the onset and modulation of key genes necessary to DA neuron development, in vivo and in vitro;
-the role of interaction with target neurons in the maturation of DA neuron function, in cell cultures and transplantation experiments.
| <= Abstract | INTRODUCTION | Materials & Methods => |
| Discussion Board | Next Page | Your Symposium |