Invited Symposium
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Results
To investigate DA neuron ontogeny, immunohistochemistry using antibodies against TH, the rate-limiting enzyme in the biosynthetic pathway of catecholamines, was used. DA midbrain neuroblasts are generated near the midbrain-hindbrain junction and migrate radially to their final position in the ventral midbrain. Rare and scattered TH-positive cells and fibers can be detected in the mouse starting at embryonic day (E) 9.5 close to the ventricular ependymal layer, suggesting that DA differentiation can occur in early postmitotic neural precursors. TH-positive clusters reminiscent of the areas A9 and A10 can be detected at E13 (Figure 2).
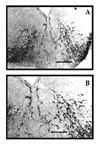
Many migrating TH-positive cells show already distinct neuronal morphological features (Figure 3).
This appears to be a distinct feature of midbrain DA neurons. For instance, in the olfactory bulb TH is not expressed until the cells have reached their final destination, the glomerular layer (18).
In vitro studies suggest that proliferation of DA neuroblasts is probably influenced by various growth factors (19). Fibroblast growth factor (FGF) 2, also known as basic FGF, and epidermal growth factor act as mitogens for neuronal precursors in fetal rat mesencephalic cultures and delay their differentiation (20). In preliminary results we find that, under these conditions, the number of TH-positive precursors appears increased and differentiation of DA neurons is enhanced by addition of Sonic Hedgehog (not shown), as in explant cultures (21). However, whilst the role of SHH in vivo is well documented, the role played by FGFs or other growth factors in the genesis of DA neurons in vivo remains to be established.
The determination of midbrain DA neurons requires the intervention of the Nurr 1 gene product immediately after induction by morphogens. Nurr 1 seems also to play a role in maintenance and plasticity of mature midbrain DA neurons. In preliminary results obtained in our laboratory we observed that in vivo Nurr 1 gene expression in rat midbrain shows a specific pattern with a peak at E13-E15. A similar pattern is reproduced in mesencephalic DA neurons in vitro, where Nurr 1 gene is highly expressed at the beginning of the cultures for two days. In addition, we have observed that the expression of Nurr 1 is plastic and can be modulated in specific culture conditions, in particular by depolarization. Retinoids also appear to modulate Nurr 1 gene expression, at least in vitro (data not shown).
Once ventral midbrain neurons have acquired a dopaminergic specification, a set of genes involved in DA functions are activated before the establishment of DA neurotransmission (Figure 4).
We have shown by immunocytochemistry and by biochemical analysis that arrival of DA fibers and detection of DA neurotrasmitter in the striatum occurs at around E15-E16 (22) (Figure 5).
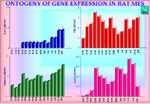
We followed the expression of various genes during rat mesencephalon ontogeny in vivo (starting from E12) (Fig 6) and in primary cultures. Amongst the various specific dopaminergic markers, TH appears early (is already present at E12). Like TH, also the synaptic vesicle monoamine transporter gene (VMAT2 ) is expressed early (14), several days before the establishment of nigrostriatal DA neurotransmission. VMAT2 belongs to the vesicular neurotransmitter transporter family and allows transport and storage of monoamines into dense core vesicles in most aminergic neurons using an electrochemical gradient. In order to terminate DA neurotransmission an highly effective mechanisms takes place to take up into the DA neurons the released dopamine. The molecule responsible for this high affinity uptake is the plasmamebrane protein dopamine transporter (DAT) (7). DAT is a member of the multigene family encoding sodium/cloride-dependent neurotransmitter transporters (23). In addition to its physiological function, DAT is the site of action of amphetamine and cocaine (23, 24) and it is responsible for the selective accumulation in DA neurons of 1-methyl-4-phenylpyridinium ion (the active MPTP catabolite). Interestingly, DAT gene transcript is detected during the ontogeny of rat ventral mesencephalon only at around E15 showing that during ontogeny, DA synthesis, storage and high-affinity uptake develop asynchronously, in a non-correlated fashion (Figure 6).
Fig. 6: Relative quantitation of DAT, TH, VMAT2 and the GABA transporter GAT 1 mRNAs in rat ventral mesencephalon during development and in adult. Values on the left represent the ratios between the yield of any given gene analyzed and the internal control HPRT. E: embryonic age; P: postnatal age; Ad: Adult.
The onset of DAT gene expression is readily followed by appearance of high affinity DA uptake in the ventral mesencephalon first and in the striatum, once the DA axon have reached this nucleus at around E16 in the rat (Figure 7).
Since the onset of DA uptake appears concomitantly with the arrival of the first DA fibers to the striatum we asked whether this event could be dependent on direct interactions of DA neurons with striatal target cells. We have addressed this question using an in vitro approach. We have shown that the level of DAT gene transcription and number of uptake sites (25, 26) are selectively increased in rodent E13 mesencephalic DA neurons in vitro after addition of E16 striatal cells in coculture (Fig. 8). More mature mesencephalic DA neuron cultures (E16) are not susceptible to the striatal influences on DAT mRNA and function (25). The latter observation suggests that mesencephalic DA neurons respond to target influences only within a restricted developmental window. Up-regulation of DAT mRNA level by striatal cells in mesencephalic DA neurons in culture seems to require direct cell interactions since target cells are ineffective when separated from mesencephalic cells by a barrier, which allows diffusion of soluble molecules (25). Interestingly, the still unidentified "signals" derived from target striatal cells appear to be specific since non-target cortical or cerebellar cells fail to stimulate DA uptake (27) or DAT gene expression (Figure 8).
Thus, DAT gene expression in developing mesencephalic DA neurons is conditioned by a specific cellular environment and probably requires continuous stimuli mediated by specific and direct cell interactions.
The striatum thus seems to regulate, both in vivo and in vitro, the maturation of the dopaminergic function during early and late development and the survival of DA neurons in postnatal life. This hypothesis is also supported by a wealth of transplant studies showing that the striatum can sustain maturation, axonal growth and survival of grafted embryonic mesencephalic DA neurons in studies carried out in the last two decades in animal models of Parkinson's disease, as well as on parkinsonian patients (28). We have used a mouse model treated with the dopaminergic neurotoxin MPTP to lesion bilaterally the substantia nigra. We have developed a method that produces a selective degeneration of the dopaminergic nigrostriatal system in mice by a combined acetaldehyde/MPTP treatment, since mice are quite resistant to MPTP alone. Lesioned or normal mice received unilateral graft of either dissociated embryonic ventral midbrain (E12-13), hypothalamic (E17-18) cells or vehicle. The animals were tested to evaluate functional recovery at various times after the operation. Four months later animals were sacrificed and the extent of DA reinnervation in the striatum and the number of surviving grafted DA neurons was evaluated. The mesencephalic neurons implanted in a lesioned host form a dense network of fibers which establish functional reinnervation of the striatum. After several months the entire striatal parenchyma appears reinnervated; on average, 20% of the grafted mesencephalic dopaminergic cells survive. Implants of embryonic hypothalamic neurons instead, show little or no survival. Moreover, the overall DA fiber outgrowth of transplanted mesencephalic DA neurons is significantly reduced when grafted in non lesioned hosts, where the intrinsic DA innervation is left intact (29) (Figure 9).
These findings suggest that in the nigrostriatal system a matching between proper targets and proper presynaptic elements is required for the maturation of embryonic dopaminergic neurons.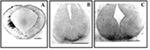 Click to enlarge
Fig. 2: Early appearance of TH-positive cells in mouse midbrain. Time course of appearance of TH-positive cells is shown by anti-tyrosine hydroxylase immunoreactivity. Tissue were fixed in 5% acrolein (15). In A is shown an early cluster of TH-positive cells at E9.5-E10. The cluster is on one side only due difficulties in early embryos orientation during cutting (15) Bar = 170 millimicron. In B TH-positive cells in ventral nuclei are shown in E10.5-11 mesencephalon. Bar = 212 millimicron. In C TH-positive clusters at E13 are appear distinct in two groups, reminiscent of A9 and A10 areas. Bar = 212 millimicron.
Click to enlarge
Fig. 2: Early appearance of TH-positive cells in mouse midbrain. Time course of appearance of TH-positive cells is shown by anti-tyrosine hydroxylase immunoreactivity. Tissue were fixed in 5% acrolein (15). In A is shown an early cluster of TH-positive cells at E9.5-E10. The cluster is on one side only due difficulties in early embryos orientation during cutting (15) Bar = 170 millimicron. In B TH-positive cells in ventral nuclei are shown in E10.5-11 mesencephalon. Bar = 212 millimicron. In C TH-positive clusters at E13 are appear distinct in two groups, reminiscent of A9 and A10 areas. Bar = 212 millimicron.
 Click to enlarge
Fig. 3: Migrating TH-positive precursors. Many TH-positive cells appear immediately after leaving the ependymal layer and migrating radially toward the ventral mesencephalon at E13 (A). Migrating TH-positive cells show clearly neurites and typical cytoplasmic immunoreactivity (B). A. Bar = 100 millimicron. B. Bar = 40 millimicron.
Click to enlarge
Fig. 3: Migrating TH-positive precursors. Many TH-positive cells appear immediately after leaving the ependymal layer and migrating radially toward the ventral mesencephalon at E13 (A). Migrating TH-positive cells show clearly neurites and typical cytoplasmic immunoreactivity (B). A. Bar = 100 millimicron. B. Bar = 40 millimicron. Click to enlarge
Fig. 4: Tentative model and time course for DA neuron development. The diagram summarizes the early events and the time course in rodent DA neurons development. The time of onset of the various genes is shown by coloured arrows. The colours indicate localization of the active gene products. D2-R, D2 type dopamine receptors; AHD2, retinoic acid-generating enzyme aldehyde dehydrogenase; VMAT2, synaptic vesicle monoamine transporter; Nurr 1 and Ptx3, transcription factors; ret, receptor tyrosine kinase c-Ret which together with GFRa1 form the receptor complex for the glial cell line-derived neurotrophic factor (GDNF); TH, tyrosine hydroxylase; DAT, dopamine transporter; E, embryonic age in days.
Click to enlarge
Fig. 4: Tentative model and time course for DA neuron development. The diagram summarizes the early events and the time course in rodent DA neurons development. The time of onset of the various genes is shown by coloured arrows. The colours indicate localization of the active gene products. D2-R, D2 type dopamine receptors; AHD2, retinoic acid-generating enzyme aldehyde dehydrogenase; VMAT2, synaptic vesicle monoamine transporter; Nurr 1 and Ptx3, transcription factors; ret, receptor tyrosine kinase c-Ret which together with GFRa1 form the receptor complex for the glial cell line-derived neurotrophic factor (GDNF); TH, tyrosine hydroxylase; DAT, dopamine transporter; E, embryonic age in days.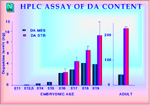 Click to enlarge
Fig. 5: High performance liquid chromatography (HPLC) assay of DA content in embryonic and adult ventral mesencephalon (mes) and striatum (str). Tissues at various ages where homogenized in 0.1 N perchloric acid, centrifuged and DA levels were determined by HPLC with electrochemical detection using a reverse phase column.
Click to enlarge
Fig. 5: High performance liquid chromatography (HPLC) assay of DA content in embryonic and adult ventral mesencephalon (mes) and striatum (str). Tissues at various ages where homogenized in 0.1 N perchloric acid, centrifuged and DA levels were determined by HPLC with electrochemical detection using a reverse phase column. Click to enlarge
Fig. 7: DA uptake during ontogenesis. The DA uptake in mesencephalic tissues was assayed in dissociated cells obtained at the various ages indicated maintained for 4 hours in vitro at 37 centigrade. Labelled DA was used at 50 nM. Blanks represents the uptake values at 4 centigrades, when active transport is blocked. Specific uptake occurs at E16, when it is signifantly different from blank values (star). Each bar represents the mean and standard deviation of separate experiments done in triplicates.
Click to enlarge
Fig. 7: DA uptake during ontogenesis. The DA uptake in mesencephalic tissues was assayed in dissociated cells obtained at the various ages indicated maintained for 4 hours in vitro at 37 centigrade. Labelled DA was used at 50 nM. Blanks represents the uptake values at 4 centigrades, when active transport is blocked. Specific uptake occurs at E16, when it is signifantly different from blank values (star). Each bar represents the mean and standard deviation of separate experiments done in triplicates. Click to enlarge
Fig. 8: RT-PCR analysis of DAT, VMAT2 and TH mRNAs levels in E13 MES cultures and cocultures. E13 ventral MES cells were grown alone, in coculture with E16 striatal cells or with E16 parietal cortex cells and cultured for various days. Values on the left represent the ratio between the yield of any given gene analyzed and that of HPRT. The values shown are obtained from three independent sister cultures (mean and standard error). Stars (*) indicate that difference between control cultures and cocultures are at least 95% significant. (ANOVA, Scheffé F -test).
Click to enlarge
Fig. 8: RT-PCR analysis of DAT, VMAT2 and TH mRNAs levels in E13 MES cultures and cocultures. E13 ventral MES cells were grown alone, in coculture with E16 striatal cells or with E16 parietal cortex cells and cultured for various days. Values on the left represent the ratio between the yield of any given gene analyzed and that of HPRT. The values shown are obtained from three independent sister cultures (mean and standard error). Stars (*) indicate that difference between control cultures and cocultures are at least 95% significant. (ANOVA, Scheffé F -test). Click to enlarge
Fig. 9: Survival and development of grafted dopaminergic neurons in adult host striata. The number TH positive neurons in mesencephalic (MES) and hypothalamic (HYP) cell suspension was detected by TH immunofluorescence prior to implantation in the host striatum. Four months after grafting TH positive neurons were counted in sections and the total number estimated, which gave the % of survival of MES and HYP after implantation in lesioned (ACE/MPTP) and control that had not received the neurotoxin cocktail (unlesioned). The maturation of TH positive neurons (Development) was assessed by comparing average cell bodies diameters, number of outgrowing neurites and, where possible, their lenght.
Click to enlarge
Fig. 9: Survival and development of grafted dopaminergic neurons in adult host striata. The number TH positive neurons in mesencephalic (MES) and hypothalamic (HYP) cell suspension was detected by TH immunofluorescence prior to implantation in the host striatum. Four months after grafting TH positive neurons were counted in sections and the total number estimated, which gave the % of survival of MES and HYP after implantation in lesioned (ACE/MPTP) and control that had not received the neurotoxin cocktail (unlesioned). The maturation of TH positive neurons (Development) was assessed by comparing average cell bodies diameters, number of outgrowing neurites and, where possible, their lenght.
<= Materials and Methods
INTRODUCTION
Discussion and Conclusion=>
| Discussion Board | Next Page | Your Symposium |
 Click to enlarge
Click to enlarge