Pharmacology & Toxicology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Results
Cell culture:
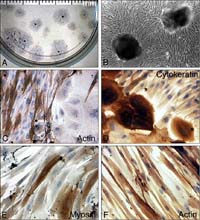
Collagen coated dishes proved to support adhesion of the explants and growth of the cells best. After 7 days in culture (div) cells with the typical elongated morphology of smooth muscle cells grew out of the attached explants (Fig.2a, b). Those cells stained positive for smooth muscle cell actin and were negative for cytokeratin (Fig. 2 c,d). Aside those cells we observed epitheloid cells, which showed cytokeratin positive immunoreactivity but were negativ for actin (Fig. 2d, c). Those cells are therefore thought to be of urothelial origin.
 Click to enlarge
Fig. 2: Morphology of explant cultures of guinea-pig urinary bladder dome. (A) low magnification view, the explants laying centrally within the hallow of outgrowing cells are about 0.5 mm in diameter. (B) higher magnification shows the typical smooth muscle cell morphology with the hill and valley growth pattern. (C, D, E) immunocytochemical staining for the antibodies as indicated in the pictures. (F) human prostate smooth muscle cells also stain positiv for smooth muscle cell actin.
Click to enlarge
Fig. 2: Morphology of explant cultures of guinea-pig urinary bladder dome. (A) low magnification view, the explants laying centrally within the hallow of outgrowing cells are about 0.5 mm in diameter. (B) higher magnification shows the typical smooth muscle cell morphology with the hill and valley growth pattern. (C, D, E) immunocytochemical staining for the antibodies as indicated in the pictures. (F) human prostate smooth muscle cells also stain positiv for smooth muscle cell actin.
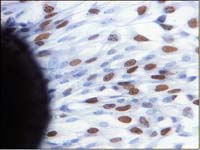
Because the state of the cells within the cell cycle might be of some impact on transmitter expression, we used BrdU pulse labelling to label those cells, which were in synthesis phase during a defined time range. As can be seen in Figure 3, about 40 % of the cells in a confluent cell layer incorporated BrdU during an 5 hours incubation.
 Click to enlarge
Fig. 3: BrdU pulse labelling experiment. Stained (brown) nuclei indicate incoporation of BrdU. Left hand an explant is seen.
Click to enlarge
Fig. 3: BrdU pulse labelling experiment. Stained (brown) nuclei indicate incoporation of BrdU. Left hand an explant is seen.
Calcium-imaging: We tested the cells routinely for their response to carbachol, nor-epinephrine, dopamine, ATP and histamine. Figure 4 presents an example of a ratio-measurement for dopamine effect on smooth muscle cells from human prostate.
 Click to enlarge
Fig. 4: Snapshots from an original recording of a dopamine experiment with smooth muscle cells cultured from human prostate. The culture had been 11 DIV. The recording was done with a ZEISS FS upright microscope using a 63x objective and the appropriate filter set for fura-2 fluorescence. Every 1 sec one single shot at an excitation wavelength of 340nm and another at 380 nm were taken at the emission wavelength of 510 nm. From these data the ratio image was calculated. 1 mM Dopamine was applied for 10 sec from a micropipette, which had been places near to the cells. Kinetics (B) were calculated as integrated values of fluorescence intensity from the areas of interest as lined out in (A). Please note the very rapid upstroke followed by a plateau of slightly elevated calcium concentrations. The calcium concentration reversed to basic levels within 10 min of wash with ringer. Cells were vital at least for 2 h under experimental conditions. (C) shows single ratio images in 5 sec intervals. Calcium concentration is color coded with blue color representing low and red color representing high calcium levels.
Click to enlarge
Fig. 4: Snapshots from an original recording of a dopamine experiment with smooth muscle cells cultured from human prostate. The culture had been 11 DIV. The recording was done with a ZEISS FS upright microscope using a 63x objective and the appropriate filter set for fura-2 fluorescence. Every 1 sec one single shot at an excitation wavelength of 340nm and another at 380 nm were taken at the emission wavelength of 510 nm. From these data the ratio image was calculated. 1 mM Dopamine was applied for 10 sec from a micropipette, which had been places near to the cells. Kinetics (B) were calculated as integrated values of fluorescence intensity from the areas of interest as lined out in (A). Please note the very rapid upstroke followed by a plateau of slightly elevated calcium concentrations. The calcium concentration reversed to basic levels within 10 min of wash with ringer. Cells were vital at least for 2 h under experimental conditions. (C) shows single ratio images in 5 sec intervals. Calcium concentration is color coded with blue color representing low and red color representing high calcium levels.
Interestingly, when comparing the different cell preparations, we found that cells from the different regions of the lower urinary tract had quite different neurotransmitter response patterns. For a semi-quantitative analysis see Table 1.
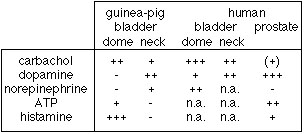 Tab. 1: Results of the calcium-imaging experiments.
Tab. 1: Results of the calcium-imaging experiments.
We tested several transmitter blockers and found, that atropine and pirenzepine completely blocked the calcium transients upon carbachol stimulation. On the other hand neither prazosine (a1-blocker) nor SCH-23390 (D1-blocker) were able to completely block the dopamine action.
| <= Materials & Methods | RESULTS | Discussion & Conclussions => |
| Discussion Board | Next Page | Your Poster Session |