Cell Biology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Results
Muscle Adenylate Levels and Maximal Na+K+-ATPase Tissue Activities
Mean ATP levels in skeletal muscle were 4.1 ± 0.3 µmol/g wet mass in euthermic S. lateralis and 30 % lower at 2.9 ± 0.3 µmol/g wet mass when measured in hibernating animals (Table 1). ADP and AMP were similarly reduced in hibernating individuals so that, overall, total adenylates decreased significantly by 32%. No change in energy charge, [ATP + 0.5 ADP] / [ATP + ADP + AMP], was observed between euthermic and hibernating states indicating that a regulated reduction in all adenylates occurred. A common product of adenylate degradation, such as during burst muscle work, is IMP which is produced by the action of AMP deaminase. However, IMP levels were not altered in the muscle of these hibernating individuals. Maximal activities of Na+K+-ATPase in homogenates from euthermic and hibernating S. lateralis are shown in Figure 3. Activity was highest in kidney with euthermic values 5-fold or more higher than activities in liver, heart and skeletal muscle. Na+K+-ATPase activities in skeletal muscle, kidney, and liver all decreased significantly during hibernation, falling to 40, 59, and 54% respectively, of the corresponding euthermic values. Activity in heart was unaffected by hibernation.
Table 1. Adenylate and IMP levels in skeletal muscle from Spermophilus lateralis under hibernating and euthermic conditions.
Euthermic Hibernator
µmol/g wet mass
-------------------------------------------------------------------------
ATP 4.1 ± 0.03 2.9 ± 0.3*
ADP 0.8 ± 0.1 0.5 ± 0.1*
AMP 0.2 ± 0.03 0.1 ± 0.03*
IMP 0.3 ± 0.1 0.2 ± 0.04
Total Adenylates 5.1 ± 0.4 3.5 ± 0.4*
Energy charge 0.87 ± 0.01 0.9 ± 0.01
-------------------------------------------------------------------------
Data are means ± SEM, n=5-8 individual samples. Energy charge is [ATP + 0.5 ADP] / [ATP +ADP + AMP]. *Significantly different from the corresponding euthermic value using the Student's t-test (2-tailed), P < 0.05.
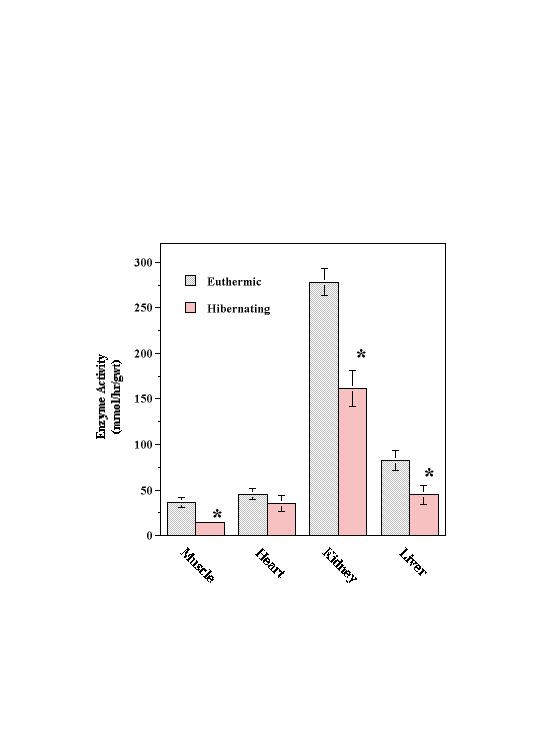 Figure 3. Maximal Na+K+-ATPase Activity in S. lateralis tissues. Values are means ± S.E.M. for n=4 determinations on separate individuals. *-Significantly different from the corresponding euthermic value, P < 0.05.
Figure 3. Maximal Na+K+-ATPase Activity in S. lateralis tissues. Values are means ± S.E.M. for n=4 determinations on separate individuals. *-Significantly different from the corresponding euthermic value, P < 0.05.
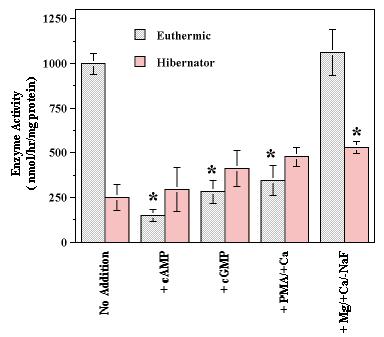 Figure 4. Effect of stimulating the activities of endogenous protein kinases on skeletal muscle Na+K+-ATPase activity in vitro. Values are means ± S.E.M. (n=4). Incubations contained 10 mM ATP, 30 mM NaF, and 15 mM MgCl2 (control) with 0.3 mM cAMP (+cAMP), 0.3 mM cGMP (+ cGMP), or 1.3 mM CaCl2 + 7 µg/mL phorbol 12-myristate 13-acetate (+ Ca2+/PMA).The final condition contained 15 mM MgCl2 and 1.3 mM CaCl2 in the absence of ATP and NaF (+Mg2+/+Ca2+/-NaF) and represents a phosphatase stimulation. *- Significantly different from corresponding control (no addition) value by the Student's t-test, P < 0.05.
Figure 4. Effect of stimulating the activities of endogenous protein kinases on skeletal muscle Na+K+-ATPase activity in vitro. Values are means ± S.E.M. (n=4). Incubations contained 10 mM ATP, 30 mM NaF, and 15 mM MgCl2 (control) with 0.3 mM cAMP (+cAMP), 0.3 mM cGMP (+ cGMP), or 1.3 mM CaCl2 + 7 µg/mL phorbol 12-myristate 13-acetate (+ Ca2+/PMA).The final condition contained 15 mM MgCl2 and 1.3 mM CaCl2 in the absence of ATP and NaF (+Mg2+/+Ca2+/-NaF) and represents a phosphatase stimulation. *- Significantly different from corresponding control (no addition) value by the Student's t-test, P < 0.05.
Putative phosphorylation and dephosphorylation of muscle Na+K+-ATPase,
Activity of the enzyme from euthermic squirrels was not affected when incubated under conditions that promoted protein phosphatase activity (minus ATP and NaF, plus Ca2+ and Mg2+) but incubations under conditions that promoted protein kinases strongly reduced euthermic activity. Conditions stimulating the activity of cAMP-dependent protein kinase (ATP + cAMP) reduced enzyme activity in muscle extracts from euthermic animals by 85% whereas incubations that promoted cGMP-dependent protein kinase or protein kinase C, reduced Na+K+-ATPase maximal activity by 72 and 65%, respectively. By contrast, when the enzyme from skeletal muscle of hibernating individuals was assessed, incubation under conditions that promoted protein kinase action had no significant effect on enzyme activity (Figure 4). However, conditions that promoted phosphatase action raised the maximal activity of the enzyme by 30%.
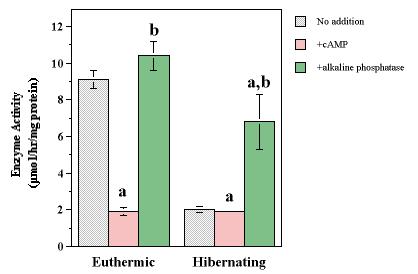 Figure 5. Phosphorylation and subsequent dephosphorylation of skeletal muscle Na+K+-ATPase from euthermic and hibernating S. lateralis. Maximal activity was measured after 2 h of in vitro incubation with no additions (no fill), after 2 h of incubation at 25°C in the presence of 10 mM ATP, 10 mM MgCl2, and 0.3 mM cAMP (hatched fill), and after a subsequent 2 h incubation of the in vitro phosphorylated enzyme in the presence of 10 U alkaline phosphatase (black fill). Values are means ± S.E.M. for n=4 separate determinations. a- Significantly different from control incubations without added effectors, P <0.05; a- significantly different from incubations that promoted in vitro phosphorylation.
Figure 5. Phosphorylation and subsequent dephosphorylation of skeletal muscle Na+K+-ATPase from euthermic and hibernating S. lateralis. Maximal activity was measured after 2 h of in vitro incubation with no additions (no fill), after 2 h of incubation at 25°C in the presence of 10 mM ATP, 10 mM MgCl2, and 0.3 mM cAMP (hatched fill), and after a subsequent 2 h incubation of the in vitro phosphorylated enzyme in the presence of 10 U alkaline phosphatase (black fill). Values are means ± S.E.M. for n=4 separate determinations. a- Significantly different from control incubations without added effectors, P <0.05; a- significantly different from incubations that promoted in vitro phosphorylation.
When muscle extracts from euthermic squirrels were incubated in vitro for 2 h in the presence of Mg.ATP + cAMP (Figure 5), Na+K+-ATPase activity declined from 9.1 U/mg protein in incubations with no additions to 1.9 U/mg protein in incubations with Mg.ATP + cAMP, a value identical to that in muscle extracts from hibernators. When the phosphorylation-treated euthermic enzyme was subsequently incubated for 2 h with alkaline phosphatase, Na+K+-ATPase activity was entirely restored, increasing 11.5-fold to 10.4 U/mg protein, a value not significantly different from the original activity. Different results occurred when membrane extracts from muscle of hibernating animals were tested. Incubation with Mg.ATP + cAMP had no measurable effect on Na+K+-ATPase activity but the subsequent treatment with alkaline phosphatase raised enzyme activity by 3.5-fold.
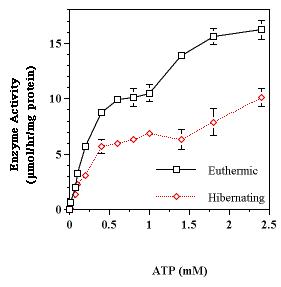 Figure 6. ATP concentration dependence of skeletal muscle Na+K+-ATPase activity from euthermic and hibernating S. lateralis. Data are means ± SEM, n = 4.
Figure 6. ATP concentration dependence of skeletal muscle Na+K+-ATPase activity from euthermic and hibernating S. lateralis. Data are means ± SEM, n = 4.
Reversible phosphorylation often alters substrate affinity of an enzyme. To determine if this was the case with Na+K+-ATPase, the velocity versus [ATP] relationship was examined for the enzyme in membrane extracts from both euthermic and hibernating muscle (Figure 6). The substrate dependence for euthermic ground squirrel Na+K+-ATPase was described by a hyperbolic curve and lacked an intermediary plateau that is characteristic of the regulating effect of ATP on the enzyme (10). However, the solubilization of this membrane bound enzyme by non-ionic detergents converts a complex substrate-velocity dependence into a hyperbolic curve (11). The ATP substrate dependency for the hibernator had a reduced maximal velocity, lacked an intermediary plateau, and did not show a change in substrate affinity. The Km values, assuming a hyperbolic relationship, for the enzymes from euthermic and hibernating squirrels were 0.60 ± 0.05 mM and 0.43 ± 0.06 mM respectively.
| <= Materials & Methods | RESULTS | Discussion & Conclussions => |
| Discussion Board | Next Page | Your Poster Session |