Invited Symposium: Stroke/Cerebral Vasospasm
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Nerves containing nitric oxide synthase (NOS) are present in the adventitial layer of major cerebral arteries and have also been observed closely associated with the basal lamina of cerebral arterioles and capillaries (1, 2). In the rat basilar artery, neuronal NOS immunoreactivity was found in 30% of all neuronal profiles identified, was located both at varicosity and intervaricosity regions, and was associated with nerves containing small spherical agranular vesicles (1). Nitrergic nerve fibres with similar morphology and distribution are present in the human middle cerebral artery (3).
VIP-immunoreactive nerve fibres have been demonstrated in human and animal cerebral arteries. They occur as non-varicose bundles or single fibres with extending branches which form a well-developed, varicose plexus at the adventitial-medial border of the major arteries (4, 5). Following field stimulation of the peripheral end of the cut facial nerve, VIP was released into the superfusate of the cat cerebral cortex, thus showing that peptidergic nerves have the potential to be involved in physiological cerebral vasodilation. (6).
The origin of these perivascular nerves has been traced to the sphenopalatine, otic and trigeminal ganglia, with considerable overlap in distribution for VIP and NOS immunoreactive neurones (7, 8). The present study examines the possibility that VIP and NOS may be localised in the same nerve fibres, and may be functionally coupled to mediate cerebral vasodilation.
Materials and Methods
Sheep middle cerebral arteries were cut into rings 4mm in length and the endothelium removed using a roughened wire. Functional denudation was confirmed by lack of relaxant effect to acetylcholine (10microM) in 5-HT precontracted vessel rings.
VIP and NOS immunocytochemistry
Immunocytochemical localisation of VIP and neuronal NOS was performed on formalin fixed, paraffin embedded sheep middle cerebral arteries. Sections (4microm thick) were mounted on polysilane coated slides, rehydrated and then treated with 3% H2O2 for 10 minutes to inhibit endogenous peroxidase. Microwave antigen retrieval was carried out in citrate buffer for 30 minutes. Non-specific sites were blocked by incubation in 20% sheep serum in tris buffered saline (TBS). Polyclonal antibody raised in rabbits to VIP (Europath Ltd) or neuronal NOS (Eurodiagnostica Ltd) diluted 1 in 50 in TBS was applied for 2 h at room temperature. Slides were washed and then incubated with biotinylated goat anti-rabbit immunoglobulin diluted 1 in 200 in TBS containing 2% sheep serum for 30 minutes at room temperature. The specimens were then incubated in peroxidase-labelled streptavidin diluted 1 in 300 in TBS for 60 minutes at room temperature, followed by 0.03% H2O2 plus 0.03% diaminobenzidine. The procedure produced a dark brown deposit at the site of antgen expression. Serial sections of artery were stained for VIP and nNOS in order to allow co-localisation of each antigen in the artery wall. Negative control slides omitted the VIP or nNOS antibody in the staining protocol.
Neurally-mediated relaxation
Each ring was suspended on two intraluminal parallel wires of 100microm diameter in a siliconized (Sigmacote) tissue bath connected to a transducer (Grass) for isometric force recording. The tissues were bathed in Krebs-Henseleit solution and bubbled with 95% O2/5% CO2 at 37oC. The composition of the Krebs-Henseleit solution was as follows (mM): NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5 and glucose 11. Sheep middle cerebral artery rings were pre-contracted with 5-HT (1-10 microM) before addition of VIP or field stimulation of the intramural nerves. VIP was added in cumulatively increasing concentrations, followed by washout, incubation with VIP antiserum for 20 min, and finally repeating the precontraction with 5-HT and concentration-response curve to VIP. Each treated artery ring was paired with appropriate time control measurements using another middle cerebral artery ring from the same head, which was subjected to an exactly parallel protocol. Electrical field stimulation (EFS) was performed using two parallel silver wire electrodes placed 2 mm apart on either side of the arterial ring, in preparations that had been precontracted using 5-HT 10 microM, and treated with guanethidine 5 microM and atropine 2 microM to prevent neurally-mediated vasoconstriction. Field stimulation (30 volts, 1 Hz frequency, 10-100 micros pulse width and 1-60 pulses per train) produced a relaxant response of 40-50% of 5-HT-induced tone, and was repeated in a 10 minute cycle. Relaxation in response to field stimulation was abolished by tetrodotoxin (100-500nM). A parallel ring was maintained as an untreated or vehicle treated time control (less than 10% variation for 10 stimulation cycles).
Assay of cyclic nucleotides
Whole sheep middle cerebral arteries were incubated in Krebs-Henseleit solution at 37oC containing zaprinast 100microM for cGMP measurements, or isobutylmethylxanthine 100microM for cAMP measurements. After 10 min incubation with 5-HT 1microM, VIP 200nM was added for 2 min (the time required for maximal relaxation). The artery was then rapidly frozen in liquid N2, and homogenized 3 times in 0.5ml of ice-cold 9% trichloroacetic acid (TCA). The homogenate was centrifuged for 10 min at 5000g. The supernatant was washed 3 times with 3 volumes of water-saturated ether, freeze-dried, and stored at -80oC. Samples were reconstituted, adjusted to pH 7.5 and their guanosine 3':5'-cyclic monophosphate (cGMP) content was determined using commercially available radioimmunoassay kits (Amersham).
Results
Immunocytochemistry
VIP and neuronal NOS immunoreactivity was identified in many branches of the adventitial nerve plexus of all the sheep middle cerebral arteries examined. Serial sections demonstrated VIP and NOS immunoreactivity in the same adventitial nerve fibres.
Relaxation of artery rings
VIP antiserum at a dilution of 1:256 significantly inhibited the VIP-induced relaxation of sheep middle cerebral artery rings at all concentrations of VIP studied (Figure 1). This was shown to be specific since rabbit pre-immune serum at the same concentration did not antagonise VIP. Furthermore, VIP antiserum had no effect on relaxations produced by CGRP. VIP antiserum (1:256) inhibited the vasodilator response following EFS (Figure 1). Inhibition of EFS-induced vasodilation produced by VIP antiserum was shown to be specific since no effect was produced by either the vehicle or by pre-immune serum. Neural relaxation of sheep middle cerebral artery rings was inhibited by L-NAPNA, a selective antagonist of NOS present in nerves (9). VIP-induced relaxation of sheep middle cerebral artery was antagonised by L-NOArg (50microM) but not by D-NOArg. VIP induced relaxation was augmented by the inhibitor of cGMP phosphodiesterase, zaprinast 10 microM (Figure 1).
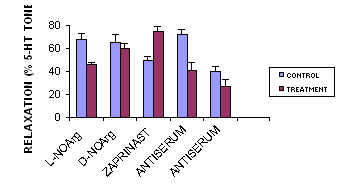
Figure 1 Precontracted sheep middle cerebral artery rings were relaxed by addition of exogenous VIP (ADDED VIP) or by stimulation of the intramural nerves (EFS). VIP-induced relaxation was inhibited by L-NOArg 50 mM but not by D-NOArg 50 mM, and was augmented by zaprinast 10 mM. VIP antiserum (1:256) inhibited both exogenous VIP and neurally-mediated relaxation. n=6, * P<0.05.
Cyclic-GMP content
The cGMP content in sheep middle cerebral artery rings was reduced 4-fold by removal of the endothelium. In endothelium-denuded vessels, VIP (200 nM) increased the cGMP levels by 2.4-fold, and this increase was inhibited by pretreament with L-NOArg but not D-NOArg. Sodium nitroprusside (100 microM) also increased the cGMP measurement in endothelium-denuded artery rings (approximately 10-fold rise).
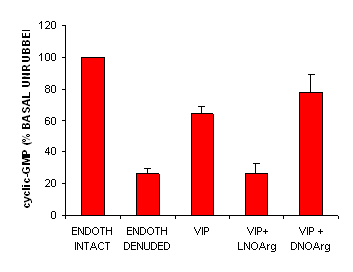
Figure 2 Cyclic-GMP content of sheep middle cerebral artery. The endothelium-intact rings contained 165+19 pmol/g wet weight. VIP 200 nM increased cyclic-GMP, which was reduced by L-NOArg 100 mM but not by D-NOArg 100 mM. n=6, * P<0.05
Discussion and Conclusion
The results presented suggest that both VIP and NO contribute to neurogenic vasodilation of cerebral arteries. Stimulation of these nerves in the sheep middle cerebral artery causes relaxation that was inhibited both by VIP antiserum and by NOS inhibitors in these experiments. The VIP antiserum treatment protocol caused quantitatively similar inhibition of the relaxant response to VIP and the relaxant response to nerve stimulation. The L-NAPNA treatment caused more than 90% inhibition of neurally-mediated relaxation. Thus the data suggests that VIP and NO are the major neurotransmitters of cerebral neurogenic relaxation, and that both of these neurotransmitters are needed for relaxation to take place.
The relaxation produced by VIP is mediated by NO, according to the present results. Thus the relaxation produced by VIP was reduced by the NOS inhibitor L-NOArg. Moreover, VIP-induced relaxation was associated with an increase in cyclic-GMP concentration and was augmented by zaprinast, a selective inhibitor of cyclic-GMP phosphodiesterase. These results in cerebral arteries contrast with the action of VIP in systemic arteries, which is mediated by cyclic-AMP
The immunocytochemical study showed that VIP and NOS are co-localised to the same nerve processes in the artery wall. Thus we propose that these nerves release VIP, which then activates NOS in the same nerves, leading to NO formation and cerebrovascular relaxation.
Acknowledgements
This work was supported by The British Heart Foundation.
References
1. Loesch A, Belai A & Burnstock G (1994) An ultrastructural study of NADPH-diaphorase and nitric oxide synthase in the perivascular nerves and vascular endothelium of the rat basilar artery. J Neurocytol 23, 49-59.
2. Iadecola C, Beitz AJ, Renno W, Xu X, Mayer B & Zhang F (1993) Nitric oxide synthase-containing neural processes on large cerebral arteries and cerebral microvessels. Brain Research 606, 148-155.
3. Gorelova E, Loesch A, Bodin P, Chadwick L, Hamlyn PJ & Burnstock G (1996) Localization of immunoreactive factor VIII, nitric oxide synthase, substance P, endothelin-1 and 5-hydroxytryptamine in human postmortem middle cerebral artery. J Anat 188, 97-107.
4. Edvinsson L & Ekman R. (1984) Distribution and dilatory effect of vasoactive intestinal polypeptide (VIP) in human cerebral arteries. Peptides; 5: 329-331.
5. Edvinsson L, Jansen I, Kingman TA & McCulloch J. (1990) Cerebrovascular responses to capsaicin in vitro and in situ. Br.J.Pharmacol.; 100, 312-318.
6. Goadsby PJ & Shelly S. (1990) High-frequency stimulation of the facial nerve results in local cortical release of vasoactive intestinal polypeptide in the anaesthetised cat. Neurosci. Lett; 112, 282-289.
7. Nozaki K, Muskowitz MA, Maynard KI, Koketsu N, Dawson TM & Bredt DS (1993) Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibres in cerebral arteries. J Cer Blood Flow Met 13, 70-79.
8. Edvinsson L, Hara H & Uddman R. (1989) Retrograde tracing of nerve fibres to the rat middle cerebral artery with true blue: co-localisation with different peptides. J.Cereb.Blood Flow Met., 9, 212-218.
9. Babbedge RC, Walace P, Gaffen ZA, Hart SL & Moore PK (1993) L-NAPNA is anti-nocicetive in the mouse. NeuroReport 4, 307-310.
| Discussion Board | Previous Page | Your Symposium |