Invited Symposium: SERCA-Type of Calcium Pumps and Phospholamban
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
SERCA pumps
Mammal and bird genomes appear to contain 3 SERCA genes, in contrast to the situation in invertebrates for which, at least up till now, no indication was found for the existence of more than one SERCA gene per genome (1). The primary transcripts of the single invertebrate SERCA gene and those of the 3 vertebrate SERCA genes are subject to alternative splicing and thus able to generate two isoforms in case of SERCA1 and SERCA2 and 3 isoforms for SERCA3. The SERCA1 gene shows a very narrow tissue-specific expression pattern, mainly confined to fast-twitch skeletal muscle fibers. SERCA2a is expressed in cardiac- and slow-twitch skeletal muscle, SERCA2b is the house-keeping isoform, found in the endoplasmic reticulum of all cells. The expression pattern of SERCA3 takes an intermediate position. It is expressed in endothelial cells, in cells of the hematopoietic lineage, in many secretory cell lines and in Purkinje neurons (2-4).
Characteristics of SERCA3
Of all SERCAs the properties of SERCA3 are the least understood and appear to deviate the most from the other members of the family. Like the other SERCA members SERCA3 is inhibited by thapsigargin. But unlike the others, it is not or can not be regulated by phospholamban (PLB), since it is lacking the corresponding cytosolic PLB interaction site (5). However the isolated membrane spanning segment of PLB or PLB variants with deletions in its cytosolic domain inhibit SERCA3 (6). In fact, no physiological regulator protein interacting with SERCA3 has been demonstrated yet, whereas SERCA2 proteins are, at least potentially, controlled by PLB and SERCA1 by the related sarcolipin (7). A possible interaction between SERCA3 and the GTP- binding protein Rap1b has been suggested in platelets (8). SERCA3 shows a remarkably low (supramicromolar) affinity for Ca2+ when expressed in COS or HEK cells, which raises questions as to its physiological role. Would it be expressed in a specific subcellular compartment with local high Ca2+ concentrations or are we ignorant of a potential regulator shifting its affinity more into the range of the other SERCAs i.e. roughly between 0.2 to 0.4 micromolar? It should be remarked that up till now no clear picture exists about the relative subcellular distribution of SERCA3 and of the housekeeping SERCA2b, although subcellular fractionation studies (9, 10) and some immunocytochemical data (11, 12) suggest a possible difference in the distribution of both pumps. There exists some confusion in the literature concerning the identity of the epitope recognized by a monoclonal antibody PL/IM430 initially raised against intracellular membranes purified from human platelets. We suggested initially that this antibody recognizes the human SERCA3 isoform (2) but later this contention was challenged and it was proposed that the monoclonal binds to another unknown member of the SERCA family (13). More recently it was circumstantially suggested that the PL/IM 430 recognized protein could be the SERCA3b splice variant (14). It is clear that, until this matter is solved, conclusions based on the use of this antibody should be interpreted with great caution.
With respect to the physiopathological implications of SERCA3 some interesting observations were made recently. Thrombocytes and endothelial cells, two cell types playing a central role in the pathogenesis of hypertension show a disturbed Ca2+ homeostasis in spontaneous hypertensive rats (SHR) and in patients with essential hypertension (15). In platelets of hypertensive subjects [Ca2+]c is increased and the platelets thereby show an increased activation. Platelets of SHR showed an increased expression of SERCA3, but not of the house keeping SERCA2b isoform (16). In endothelial cells of hypertensive subjects however decreases of [Ca2+]c have been described, which would entail in a decreased production of NO, i.e.an endothelial derived relaxing factor. SERCA3 knock-out mice appear to lack a cellular Ca2+ store controlling the endothelial NO production (17) again implicating SERCA3 in the pathogenesis of hypertension.
SERCA3 pumps have been implicated in the processes controlling both the production/secretion of insulin in the cells of the pancreatic islets of Langerhans. In a db/db mouse, a non-insulin-dependent diabetes mellitus (NIDDM) model, initial induction and subsequent oscillations of [Ca2+]c[Ca2+]c upon glucose stimulation were not observed, unlike in control islets. Further analysis showed that the SERCA3 was almost entirely absent from the db/db islets. These results and thapsigargin experiments indicated that SERCA3 might play a role in the defective insulin secretion associated with NIDDM (18). A significant reduction of SERCA3 expression was also found in cells of Goto-Kakizaki rats, a non-obese model of NIDDM (19).
T and B lymphocyte activation appears to be accompanied by a rapid down-regulation of SERCA3 expression and a concomitant up-regulation of SERCA2 both at the mRNA level and at the protein level (20). It is not entirely clear what is the exact relationship of these dramatic changes in SERCA isoform expression pattern to cell activation, but recent experimental evidence suggests that SERCA pumps might be involved in controlling the cell cycle (21, 22).
The SERCA3 pump appears to be more resistant to peroxide than SERCA2b and this may help to explain the typical distribution pattern among the different cell types (23).
Molecular Characterization of SERCA3
We have molecularly characterized the human SERCA3 gene, both at the cDNA and genomic level. A composite clone pHS3 (4547 nt) encoding human SERCA3 (later designated SERCA3a) was constructed from partial cDNA clones isolated from a Jurkat T-cell cDNA library (24). Its primary translation product consists of 999 amino acid residues and is 94.3% identical to the rat SERCA3 and 76.7% and 75.2%, respectively, to the human SERCA2a and SERCA1a isoforms. The amino acid sequences conserved in SERCA3 and other mammalian SERCAs are represented by the phosphorylation site, the regions known to bind fluorescein isothiocyanate or ATP analogues in SERCA1 and thought to be part of the ATP-binding region, as well as the hydrophobic domains that may span the sarco(endo)plasmic reticulum membranes.
The sequence divergences between SERCA3 and the other SERCAs are mainly located in the N- and C-termini. Furthermore, using rat and human SERCA3-specific antibodies, we showed that the N-terminal part of SERCA3 is highly conserved in pig, cat, rat, mouse and human (24,25), whereas a more pronounced sequence variability characterizes the C-termini of SERCA3 from these mammalian species. A monoclonal antibody, PL/IM430 was shown to inhibit Ca2+-uptake in platelet intracellular membranes and suggested to recognize either the 97 kDa SERCA3 protein (2,26) or another SERCA pump (13) in human platelets.
However, from our COS-1 cell expression studies, it appears that human SERCA3 does not display a PL/IM430-recognizable epitope (24). We can not rule out the possibility that the lack of reactivity of PL/IM430 with the expressed human SERCA3 in COS-1 cells might be due to some mutations occurring in the Jurkat but not in the normal tissue SERCA3 cDNA. Such mutations may prevent the enzyme to reach the appropriate native conformation and/or affect the epitope for PL/IM430. The comparative nucleotide sequence analysis of the human SERCA3 cDNAs from Jurkat cells and kidney (recently deposited in the EMBL/GenBank data bases; ref. 27) reveals the existence of seven single base differences. Five out of them would not change the amino acid sequence. One difference (GCC to ACC) would change an Ala673 residue in Jurkat to a Thr673 residue in kidney. However, on one copy of the genomic DNA sequence (25), we found the triplet GCC. The last mutation (ATA to ATG) encodes the change of an Ile817 residue in Jurkat to an Met817 residue in kidney.
We have found that Met817 is also encoded by the SERCA3 cDNA from K562 cells and the corresponding human genomic sequence. Moreover, this Met817 is highly conserved in rat SERCA3, human SERCA1a and SERCA2a. It will be interesting to find out whether the human SERCA3 bearing the Met817 residue can bind the monoclonal antibody PL/IM430. Besides the mentioned polymorphisms, the reported human kidney SERCA3 cDNA (27) presents a deletion of three consecutive nucleotides (AAG), which in turn, contribute to the omission of a glutamate residue from the extreme C-terminus of the pump (EMSQK in kidney instead of EEMSQK in Jurkat). This is in contradiction with the human genomic and rat cDNA sequences of SERCA3 which both encode a C-terminal tail of 6 amino acids.
The size of the human SERCA3a mRNA (detected with either a 5’-end or a 3’-end probe) is 4.8 kb in most tissues examined so far. However, when the 5’-end cDNA probe is used, two mRNA species of 4.8 and 4.0 kb, respectively, were detected only in thyroid gland and bone marrow (24). Until a cDNA clone corresponding to the 4.0 kb transcript is isolated, we can only speculate that it represents a putative SERCA3 splice variant (different from those described in the next section) or another yet unknown SERCA-like variant. The relative levels of the SERCA3 mRNA measured in 50 human adult and fetal tissues dramatically vary, with high levels of expression in thymus, trachea, salivary gland, spleen, bone marrow, lymph node, peripheral leukocytes, pancreas and colon (25). Note that the cells of these tissues are also involved in secretion processes. Such events require high cytosolic concentrations of Ca2+ and might suggest a physiological role for SERCA3, which exhibits a low apparent affinity for Ca2+.
A human genomic clone (~40 kb) encoding the 3’-region of the human SERCA3 gene, was isolated and used as a biotin-11-dUTP labeled probe to map SERCA3 gene by fluorescence in situ hybridization to human chromosomal position 17p13.3 (24). Subsequent screening of a human chromosome 17-specific cosmid library resulted in the isolation of other overlapping clones. The whole cosmid contig covering a genomic region of 90 kb, encodes the SERCA3 gene, which consists of 22 exons distributed across 50 kb of genomic DNA (25,28). So far, the rabbit (23 kb) and human (26 kb) SERCA1 genes, the crustacean Artemia franciscana (65 kb) and the fruit fly Drosophila melanogaster (7.1 kb) SERCA genes have been completely characterized. Although the estimated size of a mammalian SERCA2 gene is between 45 and 50 kb (similar to that of SERCA3), no complete exon/intron organization was ever reported. The comparative exon/intron junction analysis showed that these boundaries are well conserved between human SERCA3 and SERCA1 genes, with the exception of one boundary located between exons 8 (298 bp) and 9 (167 bp) that is found in SERCA1 gene, whereas in human SERCA3 and SERCA2 genes, the two exonic sequences are joined in one exon, i.e. exon 8 (465 bp). Recently, the first complete characterization of a SERCA2 gene became available (29) and this mouse gene shares the same feature as the human one in what concerns the existence of a large exon 8 (465 bp). Phylogenetic tree analyses based on the protein sequences of invertebrate and vertebrate SERCA pumps, together with our comparative exon/intron boundary analysis support the idea that a SERCA2 precursor and SERCA3 have diverged through gene duplication events from a common ancestor gene, prior to the duplication event leading to the currently known SERCA1 and SERCA2 genes.
The transcription initiation site of the SERCA3 gene was located 152 nt upstream of the translation initiation site. Analysis of the GC-content of an 11-kb nucleotide sequence, representing the 5’-end of the gene, showed the existence of a 1.5-kb well-defined typical “CpG" island, in which exon 1 is completely embedded. Such CpG islands are characterized by dense clustering of CpG dinucleotides and are frequently associated to the 5’-flanking region of a gene. Sequence analysis revealed no TATA element upstream of the cap site. Therefore, the transcription of SERCA3 gene appears to be driven by a GC-rich TATA-less promoter. Moreover, we have identified a sequence, CCACTGC, extending from +7 to +13 nt that matches the consensus initiator (Inr) sequence YYAN(T/A)YY, where the A represents the frequently used transcription initiation site in other genes.
Almost every Inr element described so far functions in connection with upstream Sp1-binding sites. We have identified within the CpG island of SERCA3 gene, a number of 14 putative DNA-binding sites for Sp1, of which 8 sites are found immediately upstream of the cap site between positions –267 and –39. Our functional promoter analysis (25) indicated that the GC-rich region from –135 to –31 (containing 6 putative Sp1 sites) is critical for accurate initiation of the SERCA3 gene transcription. In contrast to the SERCA2 promoter, we did not identify any thyroid-responsive elements in the 5’-end of the SERCA3 gene. The existence of a TATA- Inr+ promoter (like in SERCA3 gene) appears to be prevalent among the hematopoietic lineage-specific genes and this fits well with part of the observed tissue expression pattern of SERCA3. On the other hand, TATA+ Inr- promoters (like in SERCA2 gene) may be responsible for a lineage-independent expression.
Alternative Splicing of SERCA3 pre-mRNA
In contrast to SERCA1 and SERCA2 genes, there were no indications that the SERCA3 primary transcript is also alternatively spliced until very recently, when two mouse nucleotide sequences encoding SERCA3a (corresponding to the reported rat and human SERCA3) and SERCA3b have been deposited in the EMBL/GenBank data bases (30), but without any indication concerning the alternative processing mechanisms. Moreover, it appears that this picture of the SERCA3 isoform diversity was still incomplete, since we are now able to distinguish three SERCA3 isoforms both in human and mouse (25). In both genes, exon 21 is alternatively used and can be excluded, included (using the 5’-donor splice site D2) or partially retained due to existence of an internal 5’-donor splice site (D1 site).
It is interesting to note that although the same alternative splicing mechanism applies for both species (Fig.1), the length of the optional exon (exon 21) is different in human (101 bp) and in mouse (86 bp) SERCA3 genes. The human exon 21 is 15 nt longer than its mouse counterpart because an additional 3’-acceptor splice site is found in the human sequence 15 nt upstream of the site corresponding to the acceptor splice site used in mouse. The translation of the human 15-nt sequence representing the 5’-end of the exon 21 introduces a new in-frame stretch of 5 amino acids ACLYP998, intercalated between the protein sequences encoded by exon 20 and the rest of exon 21. The functional significance, if any, of the ACLYP sequence remains to be further investigated.
Partial inclusion of exon 21 (88 bp in human and 73 bp in mouse) in the mRNA results in the translation of the SERCA3b isoforms (1043 aa in human and 1038 aa in mouse). Total retention of exon 21 gives rise to SERCA3c isoforms (1024 aa in human and 1021 aa in mouse). When exon 21 is skipped, SERCA3a isoforms are generated (999 aa in both human and mouse). SERCA3 isoforms differ solely in their C-terminal parts inserted downstream of amino acid 993 (which also represents the point of divergence among the related SERCA1 and SERCA2 isoforms).
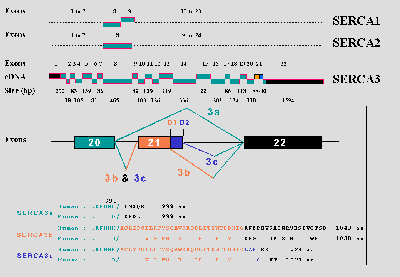 Fig.1:Comparative exon/intron organization of SERCA1, SERCA2, and SERCA3 genes (upper part) and a schematic illustration of the alternative splicing of the SERCA3 pre-mRNA (lower part).
Fig.1:Comparative exon/intron organization of SERCA1, SERCA2, and SERCA3 genes (upper part) and a schematic illustration of the alternative splicing of the SERCA3 pre-mRNA (lower part).
So far, SERCA3b and SERCA3c were found to be always co-expressed with SERCA3a in mouse pancreatic islets and in human kidney. Recently, the expression of SERCA3c in human kidney was confirmed by another group (27). Mouse SERCA3 isoforms were overexpressed in COS-1 cells and Ca2+ -uptake studies were performed on microsomes isolated from COS cells transfected with the corresponding cDNAs. We demonstrated that each SERCA3 isoform can function as a Ca2+ pump. We confirmed that SERCA3a displays indeed a lower apparent affinity for calcium ions than SERCA2b and, finally, reported that SERCA3b and SERCA3c seem to exhibit still lower Ca2+ apparent affinities than SERCA3a (2). We proposed that that the extended tails of SERCA3b and SERCA3c may directly interact with other domains of the pump, shifting the equilibrium between the enzyme E1 (binds Ca2+ with high affinity) and E2 (low affinity for Ca2+) conformational states more toward the E2 conformation.
In the past recent years, increasing information regarding SERCA3 identification, expression, molecular characterization, isoform diversity was accumulated. The current resources include SERCA3 cDNAs from rat (only for SERCA3a), mouse and human, genomic clones for human and mouse SERCA3 genes, and specific anti-SERCA antibodies generated in our lab (N89, C90 and C91). Note that a commercially available antibody against SERCA3 (ABR, Golden, CO, USA, #PA1-910) actually corresponds to our previously developed N89 antibody (2).
References
- Wuytack, F, Raeymaekers, L, Eggermont, J, Van Den Bosch, L. Verboomen, H., Mertens, L. (1998) Isoform diversity and regulation of organellar-type Ca2+-transport ATPases. Advances in Molecular and Cell Biology 23A, 205-248
- Wuytack, F, Papp, B, Verboomen, H, Raeymaekers, L, Dode, L, Bobe, R, Enouf, J, Bokkala, S, Authi, KS, Casteels, R. (1994) A sarco/endoplasmic reticulum Ca2+-ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells and in mast cells. J. Biol. Chem. 269: 1410-1416
- Wuytack, F, Dode, L, Baba-Aissa, F, Raeymaekers, L (1995) The SERCA3-type of organellar Ca2+ pumps. Bioscience Reports 15: 299-306
- Wu, KD, Lee, WS, Wey, J, Bungard, D, Lytton, J (1995) Localization and quantification of endoplasmic reticulum Ca2+-ATPase isoform transcripts. Am. J. Physiol. 269:C775-C784
- Toyofuku, T, Kurzydlowsky, K, Tada, M, MacLennan, DH (1994) Amino acids Lys-Asp-Asp-Lys-Pro-Val402 in the Ca2+-ATPase of cardiac sarcoplasmic reticulum are critical for functional association with phospholamban. J. Biol. Chem. 269: 22929-22932
- Kimura, Y, Kurzydlowski, K, Tada, M, MacLennan, DH (1996) Phospholamban regulates the Ca2+-ATPase through intramembrane interactions. J. Biol. Chem. 271: 21726-21731
- Odermatt, A, Becker, S, Khanna, VK, Kurzydlowsky, K, Leisner, E, Pette, D, MacLennan, DH (1998) Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 273: 12360- 12369
- Lacabaratz-Porret, C, Corvazier, E, Kovacs, T, Bobe, R, Bredoux, R, Launay, S, Papp, B, Enouf, J (1998) Platelet sarco/endoplasmic reticulum Ca2+-ATPase isoform 3b and Rap1b: interrelation and regulation in physiopathology. Biochem. J. 332: 173-181
- Rooney, E, Meldolesi, J (1996) The endoplasmic reticulum in PC12 cells. Evidence for a mosaic of domains differently specialized in Ca2+ handling. J. Biol. Chem. 271: 29304-29311
- Vanlingen, S, Parys, JB, Missiaen, L, De Smedt, H, Wuytack, F, Casteels, R (1997) Distribution of inositol 1,4,5-trisphosphate receptor isoforms, SERCA isoforms and Ca2+-binding proteins in RBL-2H3 rat basophilic leukemia cells. Cell calcium 22: 475-486
- Lee, MG, Xu, X, Zeng, W, Diaz, JK Wuytack, F, Kuo, TH, Raeymaekers, L, Muallem, S (1997) Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. J. Biol. Chem. 272: 17771-15776
- Kovacs, T, Berger, G, Corvazier, E, Paszty, K, Brown, A, Bobe, R, Papp, B, Wuytack F, Cramer, EM, Enouf, J (1997) Immunolocalization of the multi-sarco/endoplasmic reticulum Ca2+-ATPase system in human platelets. Br. J. Haematol. 97: 192-203
- Kovacs, T, Corvazier, E, Papp, B, Magnier, C, Bredoux, R, Enyedi, A, Sarkadi, B, Enouf, J (1994) Controlled proteolysis of Ca2+-ATPases in human platelet and non muscle cell membrane vesicles. Evidence for a multi-sarco/endoplasmic reticulum Ca2+-ATPase system J. Biol. Chem. 269 :6177-6184
- Bobe, R, Lacabaratz-Porret, C, Bredoux, R, Martin, V, Ozog, A, Launay, S, Corvazier, E, Kovacs, T, Papp, B, Enouf, J (1998) Expression of two isoforms of the third sarco/endoplasmic reticulum Ca2+-ATPase (SERCA3) in platelets. Possible recognition by PL/IM430 monoclonal antibody. FEBS Letters 423: 259-264
- Aviv, A (1996) Recent advances in cellular Ca2+ homeostasis: implications to altered regulations of cellular Ca2+ and Na+-H+ exchange in essential hypertension. Curr. Opin. Cardiol. 11: 477-482
- Papp, B, Corvazier, E, Magnier, C, Kovacs, T, Bourdeau, N, Levy-Toledano, S, Bredoux, R, Levy, B, Poitevin, P, Lompre, AM, Wuytack, F, Enouf, J. (1993) Spontaneously hypertensive rats and platelet Ca2+-ATPases: specific up-regulation of the 97-kDa isoform. Biochem. J. 295: 685-690
- Liu, LH, Paul, RJ, Sutliff, RL, Miller, ML, Lorenz, JN, Pun RY, Duffy, JJ, Doetschman, T, Kimura, Y, MacLennan, DH, Hoying, JB, Shull, GE (1997) Defective endothelium-dependent relaxation of vascular smooth muscle and endothelial cell Ca2+ signaling in mice lacking sarco(endo)plasmic reticulum Ca2+-ATPase isoform 3. J. Biol. Chem. 272: 30538-30545
- Roe, MW, Philipson, LH, Frangakis, CJ, Kuznetsov, A, Mertz, RJ; Lancaster, ME, Spencer, B, Worley, JF-3rd, Dukes, ID (1994) Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J. Biol. Chem. 269: 18279-18282
- Varadi, A, Molnar, E, Ostenson, CG, Ashcroft, SJ (1996) Isoforms of endoplasmic reticulum Ca2+-ATPase are differentially expressed in normal and diabetic islets of Langerhans. Biochem. J. 319: 521-527
- Launay, S, Bobe, R, Lacabaratz-Porret, C, Bredoux, R, Kovacs, T, Enouf, J, Papp, B (1997) Modulation of endoplasmic reticulum calcium pump expression during T lymphocyte activation. J. Biol. Chem. 272: 10746-10750
- Kuo, TH, Liu, BF, Yu, Y, Wuytack, F, Raeymaekers, L, Tsang, W (1997) Coordinated regulation of the plasma membrane calcium pump and the sarco(endo)plasmic reticular calcium pump genes by Ca2+. Cell Calcium 21: 399-408
- Waldron, RT, Short AD, Meadows, JJ, Ghosh, TK, Gill, DL (1994) Endoplasmic reticulum Ca2+ pump expression and control of cell growth. J. Biol. Chem. 269: 11927-11933
- Grover, AK, Samson, SE, Misquitta, CM (1997) Sarco(endo)plasmic reticulum Ca2+-pump isoform SERCA3 is more resistant than SERCA2b to peroxide. Am. J. Physiol. 273: C420-C425
- Dode, L, Wuytack, F, Kools, PFJ, Baba-Aissa, F, Reaymaekers, L, Briké, F, Casteels, R (1996) cDNA cloning, expression and chromosomal localization of the human sarco/endoplasmic reticulum Ca2+ -ATPase 3 gene. Biochem. J. 318:689-699
- Dode, L, De Greef, C, Mountian, I, Attard, M, Town, MM, Casteels, R, Wuytack, F (1998) Structure of the human sarco/endoplasmic reticulum Ca2+ -ATPase 3 gene. Promoter analysis and alternative splicing of the SERCA3 pre-mRNA. J. Biol. Chem. 273:13982-13994
- Bokkala, S, El-Daher, SS, Kakkar, VV, Wuytack, F, Authi, KS (1995) Localization and identification of Ca2+-ATPases in highly purified human platelet plasma and intracellular membranes. Evidence that the monoclonal antibody PL/IM430 recognizes the SERCA3 Ca2+ ATPase in human platelets. Biochem. J. 306:837-842
- Poch, E, Leach, S, Snape, S, Cacic, T, MacLennan, DH, Lytton, J (1998) EMBL/GenBank data bank accession numbers AF068220 and AF068221
- Dode, L, De Greef, C, Mountian, I, Casteels, R, Wuytack, F (1998) Characterization of the human sarco/endoplasmic reticulum Ca2+ -transport ATPase 3 gene: The promoter analysis and the alternative splicing of the SERCA3 primary transcript. Pflügers Archiv Europ. J. Physiol. 435:R8
- Ver Heyen, M, Van Baelen, K, Raeymaekers, L, Wuytack, F, (1998) Genomic structure of the mouse SERCA2 gene. Pflügers Archiv Europ. J. Physiol. 435:R21
- Tokuyama, Y, Chen, X, Roe, MV, Bell, GI (1996) EMBL/GenBank data bank accession numbers U49393 and U49394
| Discussion Board | Previous Page | Your Symposium |