Invited Symposium: Molecular and Cellular Analysis of Dopamine and Serotonin Transporters
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Impairment in the uptake of neurotransmitters results with the extracellular accumulation of the neurotransmitter, leading to profound physiological consequences. Blocking dopamine transporter by cocaine, e.g., enhances locomotion and induces behavioral and psychological changes. As to the serotonin transporter, blockers of this carrier are commonly used antidepressive drugs. Understanding the molecular mechanisms controlling the expression and function of these transporters has, therefore, implications to the normal activity of various neuronal pathways, as well as to several diseased states.
The reuptake of neurotransmitters is subject to physiological regulation, but little is known about the transcriptional and posttranslational systems involved in regulating the expression of transporters. A common structural feature of neurotransmitter transporters is the presence of phosphorylation sites for protein kinase A, protein kinase C, and Ca-calmodulin protein kinase (1,2). Experiments with various cell types indeed showed that cholera toxin, which activates cyclic-AMP production, modulates serotonin uptake (3-5), whereas an increased protein kinase C activity upon treatment with TPA down-regulates serotonin transport (6,7). As this effect of TPA was immediate, within 10-120 min, recompartmentation, changes in ion flow, or indirect phosphorylation of the transporter by TPA-induced protein kinase C was considered (8,9). In the course of the present study, it has been observed that TPA also has a long-term influence on the activity of the serotonin transporter. It was of interest, therefore, to characterize the long-term effect of TPA.
The role of N-linked glycosylation in controlling the function and stability of monoamine transporters was demonstrated for GABA (10) and catecholamine (11,12) transporters. Regional, cell, or species-specific variations in glycosylation have been shown for dopamine or SERT transporters (13-15). In insect cells glycosylation was important for stability of SERT in the membrane, but not for transport or binding activity (16). Herein we summarize experiments aimed to shed light on the regulation of SERT under various conditions, including RT-PCR analysis of the effect of the glucocorticoid hormone dexamethasone on TPA-treated cells.
Materials and Methods
Two peptides, PCGDIRMNAV and TSAGDEASHS, corresponding to amino acids 598-607 (C-terminal) and 30-39 (N-terminal) of rSERT, were coupled to KLH carrier protein. New Zealand White rabbits were immunized by intradermal injections, and booster injections were similarly administrated every 2-3 weeks. Rabbits were bled 8-10 days after the boost, IgG were precipitated by 75% ammonium sulfate, dissolved in PBS and dialyzed against PBS. For immunoblot analysis of SERT, cells were washed three times with ice-cold PBS and solubilized in 300 µl of ice-cold RIPA buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 1% TritonX-100, 1% Na deoxycholate) supplemented with 1 mM PMSF and 10 mM leupeptin. Extracts were centrifuged (15000 g 10 min), protein content in the supernatant was determined by Lowry protein assay, with BSA as a standart, and proteins were analysed on 8% SDS-PAGE. Gels were blotted to 0.45µ nitrocellulose at 4°C for 16 hr at 30V (transfer buffer: 25 mM Tris, 192 mM glycine and 20% methanol) and stained with 0.1% Ponceau S solution in 5% acetic acid to verify the transfer. Membranes were ‘blocked’ for 2 hr (10% non-fat milk in PBS), probed with antiserum (1:100 in PBS/5% milk) for 1hr at 22 °C, and rinsed (5 times, 10 min each at 22°C in PBS, 0.5% Tween-20). Membranes were then incubated with 125I-protein A (0.5 mCi/ml) in PBS/5% milk, rinsed, and autoradiographed with Kodak film.
Human choriocarcinoma placental cells (JAR) were cultured in DMEM with 10% fetal calf serum in a 10% CO2 humidified incubator at 37°C, and subcultured every 3-5 days. For uptake experiments 5x104 cells were seeded per well in 1 ml culture medium, in 24-well Costar or Falcon plates, and cells were treated 24-72 hr after seeding for the indicated period.
Cloned rSERT cDNA was kindly provided by Dr. R. Blakley. The original plasmid was used to prepare the vector pCMV-rSERT. In situ staining with X-Gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside) showed best transfection efficiency with 1 or 2 µg of plasmid DNA, incubated with cells for 16-24 hr in 2 ml plate, using the calcium phosphate method (without glycerol). For an internal control and to correct the uptake data according to transfection efficiency, pCMV-rSERT was cotransfected with pCMV-b-Gal.
RT-PCR of SERT and GAPDH (for semi-quantitative analysis) was conducted with total RNA, using MoMuLV reverse transcriptase. The primers for SERT were 5’-GAACGGGAGA-CCTGGGGCAAGAAG-3’ and 5’-GAACATCATCCCTGCCTCTACTGG-3’.
Results
Anti-SERT antibodies detecting SERT in JAR and Transfected cell - Antibodies against two synthetic peptides corresponding to the N or C terminals of rSERT were prepared. Immunoblot analysis revealed that both antibodies recognize rSERT and hSERT. The C terminal peptide PCGDIRMNAV produced a high titer of antibodies, and was used therefore for immunohistochemical studies in the rat brain (17), and for the following experiments.
Two immunoreactive protein bands of 77±3 and 95±3 kDa were observed by immunoblot analysis of JAR cell membranes (Fig. 1). A similar analysis of pCMV-rSERT-transfected COS-7 and NMB cells, previously reported to exhibit potent 3H-5HT uptake (18), showed two immunoreactive proteins, with apparent size of 80±3 and 115±3 kDa (data not shown). These proteins were absent in extracts of non-transfected cells, and when the antigen peptide was preincubated with the antibodies. Also, these antibodies failed to recognize the dopamine transporter of non-transfected NMB cells. Interestingly, the intensity of the immunoreactive bands in transfected NMB cell extracts was about 20-fold of COS-7 cells, whereas 5HT uptake in both transfected cells was similar.
Glycosylation - To further understand the nature of size variability of SERT in different cells, and to analyse the contribution of glycosylation to protein mobility and function, we examined the effect of the N-linked glycosylation inhibitor tunicamycin. Treatment of JAR and pCMV-rSERT-transfected NMB cells with 10 µg/ml tunicamycin for 24 hr resulted in 40% decrease in serotonin uptake in both cell types (Fig. 1A). The reduction in the transporter activity was correlated with a similar time-dependent decrease in the intensity of both immunoreactive proteins in JAR and transfected NMB cells (Fig. 1B, data shown for JAR cells only). By 24 hr after treatment a broad protein band of 46-51 kDa was observed (Fig. 1B), apparently representing non-glycosylated SERT.
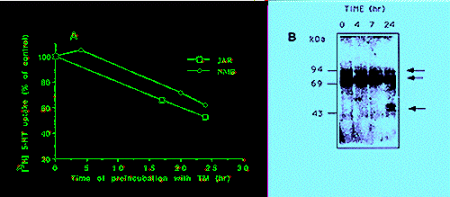 Fig: A. Tunicamycin effect (10 µg/ml) on 3H-5HT uptake in JAR and pCMV-rSERT-transfected NMB cells, conducted as described (18). B. Immunoblot analysis of JAR cells treated with tunicamycin (10 µg/ml) for the times indicated. Arrows - hSERT immunoreactive bands.
Fig: A. Tunicamycin effect (10 µg/ml) on 3H-5HT uptake in JAR and pCMV-rSERT-transfected NMB cells, conducted as described (18). B. Immunoblot analysis of JAR cells treated with tunicamycin (10 µg/ml) for the times indicated. Arrows - hSERT immunoreactive bands.
Long-term Effect of TPA on JAR Cells - Early studies showed that TPA inhibits serotonin uptake in endothelial cells (6) and platelets (7), and this was confirmed more recently with other cell lines (8,9). In these systems TPA has a rapid effect, taking a peak within 30-60 min. Fig. 2A shows that incubating the human placental JAR cells with TPA for 30 min to 72 hr has a dual effect on 3H-5HT uptake; within the first 30 min TPA decreased the uptake, but then there is a time-dependent increase in uptake. This long-term effect of TPA was also dose-dependent (Fig. 2B).
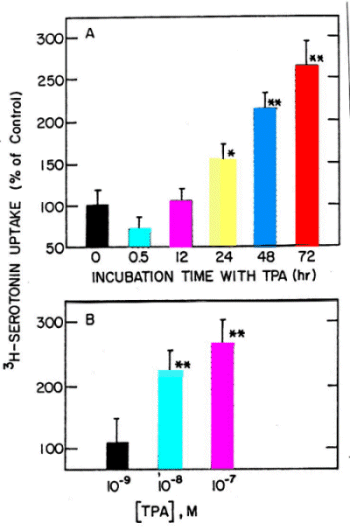 Fig. 2: Time (A) and dose (B) dependent effect of TPA on 3H-5HT uptake in JAR cells. A- 10-7 M TPA. B- Treatment for 48 hr. Data are mean±S.D. * and ** were significantly different from control, P<0.01 and 0.001, respectively.
Fig. 2: Time (A) and dose (B) dependent effect of TPA on 3H-5HT uptake in JAR cells. A- 10-7 M TPA. B- Treatment for 48 hr. Data are mean±S.D. * and ** were significantly different from control, P<0.01 and 0.001, respectively.
It was essential to evaluate whether the response of JAR cells to long-term treatment with TPA was specific. Two TPA related compounds that do not stimulate protein kinase C activity were tested. Fig. 3A shows the comparison between TPA, phorbol, and 4-a-phorbol-12-myristate-13-acetate; only TPA enhanced 3H-5HT uptake in a significant and dose-dependent manner. Lineweaver-Burk analysis (Fig. 3B) showed that 72 hr treatment with 10-7 M TPA increased the Vmax from 3.67±0.6 to 9.72±1.2, with a non-significant effect on the Km (330±33 and 411±51 in control and TPA-treated cells, respectively). Treatment of JAR cells with 5x10-5M IBMX increased 3H-5HT uptake by 136±44%, as did cholera toxin (3-5). This is in line with the notion that activation of adenylate cyclase, and protein kinase A, increase serotonin uptake (3-5). However, long-term treatment with both cholera toxin and TPA did not have an additive effect (Fig 3C), suggestive of cross-talk between common pathways, or a sequential activation of the two protein kinases.
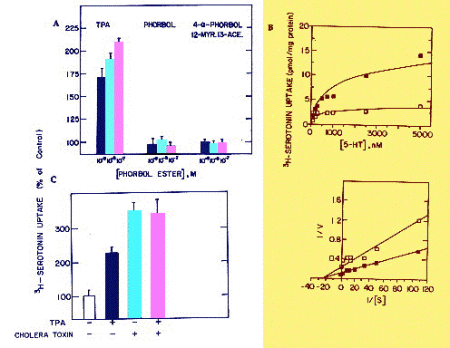 Fig. 3: A. Specificity of TPA effect. JAR cells were treated for 48 hr with different concentrations of TPA or TPA analogues. Data are mean±S.D. B. Lineweaver-Burk analysis of TPA effect on 3H-5HT uptake in untreated or TPA-treated (10-7 M for 72 hr) JAR cells. Different concentrations of unlabeled 5HT were used to increase total 5HT concentration. C. Effect of treatment with TPA (10-7 M for 48 hr) and cholera toxin (100 ng/ml for 72 hr).
Fig. 3: A. Specificity of TPA effect. JAR cells were treated for 48 hr with different concentrations of TPA or TPA analogues. Data are mean±S.D. B. Lineweaver-Burk analysis of TPA effect on 3H-5HT uptake in untreated or TPA-treated (10-7 M for 72 hr) JAR cells. Different concentrations of unlabeled 5HT were used to increase total 5HT concentration. C. Effect of treatment with TPA (10-7 M for 48 hr) and cholera toxin (100 ng/ml for 72 hr).
The long-term treatment with TPA increased SERT immunoreactivity (data not shown) and SERT mRNA levels, assessed with RT-PCR (Fig. 4). Co-treatment of JAR cells with TPA and the glucocorticoid hormone dexamethasone showed that TPA effect was inhibited by the hormone, both at the protein (not shown), and mRNA levels (Fig. 4). The effect of dexamethasone by itself was inconsistent.
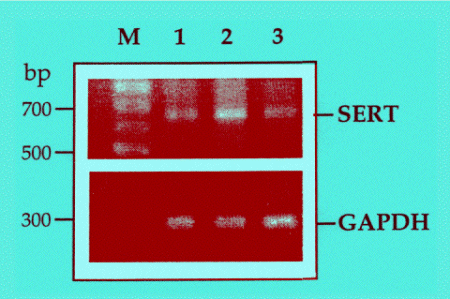 Fig. 4: Semi-quantitative RT-PCR analysis of SERT and GAPDH in JAR cells treated with TPA; inhibition by dexamethasone. Untreated cells (lane 1), cells treated for 36 hr with
10-7 M TPA (lane 2), or 10-7 M TPA and 10-8 M dexamethasone (lane 3).
Fig. 4: Semi-quantitative RT-PCR analysis of SERT and GAPDH in JAR cells treated with TPA; inhibition by dexamethasone. Untreated cells (lane 1), cells treated for 36 hr with
10-7 M TPA (lane 2), or 10-7 M TPA and 10-8 M dexamethasone (lane 3).
Discussion and Conclusion
Cloning of the serotonin transporters have shown that these glycoproteins possess consensus protein kinase A, protein kinase C, and Ca-calmodulin kinase sites (1,2), and studies with various cell types have indicated that both TPA and cyclic-AMP inducers control the expression and activity of serotonin transporters (3-9).
In addition to the short-term effect of TPA, which is inhibitory in many systems, our study shows that TPA can also increase serotonin transport. This stimulatory effect has been detected in JAR cells expressing the transporter constitutively, and in NMB and COS-7 cells transfected transiently with a pCMV vector expressing the rSERT cDNA. It is of interest, however, that TPA effect in these two systems is quite different; the phorbol ester has a modest but immediate effect on the transfected cells, whereas the stimulation of serotonin uptake in JAR cells is a slow process that continues to increase even after 48 hr. One of the interpretations of these data is that the immediate increase in serotonin uptake in transfected cells, as well as the down regulation reported previously, involve changes in the transporter protein, e.g., by indirect phosphorylation or compartmentation (8,9), whereas the slow response of JAR cells to TPA results from an increase the number of transporter molecules. Indeed, we have shown that in JAR cells the long-term treatment with TPA increased the Vmax of serotonin uptake, SERT immunoreactivity, and SERT mRNA levels determined with RT-PCR. As the long-term effect of TPA and cholera toxin was not additive, cross-talk between common pathways or sequential activation of protein kinase A and protein kinase C may take place (see 9).
The long-term effect of TPA apparently involves changes at the level of transcription, and it is likely that early-response genes such as the AP-1 complex of Jun/Fos are induced, like in other systems (19). In line with this notion, we showed herein a functional antagonism between TPA and dexamethasone. Similar antagonism between AP-1 factors and glucocorticoids was reported before (20,21). The serotonin transporter in JAR cells may therefore represent another case of cross-talk between AP-1 and glucocorticoid transcription pathways. It is of a special interest that glucocorticoid hormones appears to have different effect on the expression of serotonin transporters in young and aged animals, and in certain part of the brain, versus blood platelets (22). The implications of these observations regarding the regulation of serotonin transporters under various drug treatments, or pathological condistions such as depression, should be further analyzed.
Acknowledgments: We thank Dr. R. Blakley for the rSERT cDNA. The study was supported by the Israel Antidrug Authority and the Israel Ministry of Health.
References
- Amara S.G. and Kuhar M.J. (1993) Annu. Rev. Neurosci. 16: 73-93.
- Lesch K.P., Wolozin B.L., Murphy D.L. and Riederer P. (1993) J. Neurochem. 60: 2319-2323.
- Cool D.R., Leibach F.H., Bhalla V.K., Mahesh V.B. and Ganapathy V. (1991) J. Biol. Chem. 266:15750-15757.
- King S.C., Tiller A.A., Chang A.S. and Lam D.M.K. (1992) Biochem. Biophys. Res. Commun. 183: 487-491.
- Ramamoorthy S., Cool D.R., Mahesh V.B., Leibach F.H., Melikian H. E., Blakely R.D. and Ganapathy V. (1993) J. Biol. Chem. 268: 21626-21631.
- Myers C.L., Lazo J.S. and Pitt B.R. (1989) Am. J. Physio. 257: L253-L258.
- Anderson G.M. and Horne W.C. (1992) Biochim. Biophys. Acta. 1137: 331-337.
- Qian Y., Galli A., Ramamoorthy S., Risso S., DeFelice L.J. and Blakely R.D. (1997) J. Neurosci. 17: 45-57.
- Sakai N., Sasaki K., Nakashita M., Honda S., Ikegaki N. and Saito N. (1997) J. Neurochem. 68: 2618-26-24.
- Keynan S., Suh Y-J., Kanner B.I. and Rudnick G. (1992) Biochemistry. 31:1974-1979.
- Zhu J. and Hexum T.D. (1992) Neurochem. Int. 21: 521-526.
- Melikian H.E., McDonald J.K., Gu H., Rudnick G., Moore K.R. and Blakely R.D. (1994) J. Biol. Chem. 269: 12290-12297.
- Lew R., Melikian H.E., Patel A., Vaughan R.A., Wilson A. and Kuhar M.J. (1992) Brain Res. 584: 266-271.
- Patel A., Uhl G. and Kuhar M.J. (1993) J. Neurochem. 61: 496-500.
- Qian Y., Melikian H.E., Rye D.B., Levey A.I. and Blakely R.D. (1995) J. Neurosci. 15: 1261-1274.
- Tate C.G. and Blakely R.D. (1994) J. Biol. Chem. 269: 26303-26310.
- Amir S., Robinson B., Ratovitski T., Rea M.A., Stewart J. and Simantov R. (1998) Neuroscience 84: 1059-1073.
- Ratovitski T., Tauber M., Tafet G.E. and Simantov R. (1997) Pharmacol. Rev. Commun. 8: 245-250.
- Karin M. and Smeal T. (1992) Trends in Biochem. Sci. 17: 418-422.
- Yang-Yen H.-F. Chambard J. -C, Sun Y.-L., Smeal T., Schmidt T. J., Drouin J., and Karin M. (1990) Cell 62: 1205-1215.
- Schule R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M. and Evans R.M. (1990) Cell 62: 1217-1226.
- Slotkin T.A., McCook E.C., Ritchie J.C., Carroll B.J. and Seidler F.J. (1997) Biol Pschiatry 41: 172-183.
| Discussion Board | Previous Page | Your Symposium |