Invited Symposium: Behaviour-Induced Neural Events after Brain Injury
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
This poster is based on the work described in the 6 articles listed in the references whose co-authors are too numerous to mention in the authorship of this presentation.
Constraint-Induced (CI) Movement Therapy
A new approach to the rehabilitation of movement after stroke, Constraint-Induced (CI) Movement Therapy, has been derived directly from basic research with monkeys given somatosensory deafferentation. After unilateral forelimb deafferentation, monkeys do not use the affected limb in the free situation. However, they can be induced to use the deafferented extremity by either of two general techniques: 1) restricting movement of the intact limb for 7 or more days, 2) training the deafferented arm. A useless limb is thereby converted into a limb that can be used extensively.
The same techniques are effective for producing a substantial rehabilitation of movement after stroke in humans. CI consists of a family of therapies; their common element is that they induce stroke patients to greatly increase the use of an affected upper extremity for many hours a day over a period of 10-14 consecutive days. The signature intervention involves motor restriction of the less affected upper extremity in a sling and training of the more affected arm. The therapies result in large changes in amount of use of the more affected arm in the activities of daily living outside the clinic that persist for at least the two years measured to date. At least 50% of the chronic stroke population with motor deficit appears to be amenable to substantial improvement through the application of CI Therapy. This laboratory has recently found that the CI Therapy approach produces as good a result with the lower extremity (9 chronic stroke subjects, 2 chronic incomplete spinal cord-injured subjects) as with the upper extremity (84 subjects). Pilot work indicates that CI Therapy is also effective for the rehabilitation of upper extremity use in patients with sub-acute stroke (14 patients, 3-6 months post-stroke; Taub, Wolf, Giuliani, Light, Kulkulka, Nichols & Winstein, unpublished data).
CI Therapy-Induced Cortical Reorganization
It has been shown in monkeys who were surgically given an ischemic infarct in the cortical area controlling the movements of a hand, that training of the more affected limb and constraint of the less affected arm results in cortical reorganization so that the area surrounding the infarct not normally involved in control of the hand comes to participate in that function (Nudo, Wise, SiFuentes, & Milliken, 1996). The two studies presented here indicate that CI Therapy produces a large use-dependent cortical reorganization in humans with stroke-related hemiparesis of an upper extremity. To the best of our knowledge, these two studies are the first to demonstrate an alteration in brain structure or function associated with therapy-induced rehabilitation of movement after CNS damage in humans.
Materials and Methods
Study 1: Transcranial Magnetic Stimulation
Focal transcranial magnetic stimulation (TMS) was used to map the areas of the brain that control arm movement in 6 patients with a chronic upper extremity hemiparesis before and after CI Therapy (Liepert, Miltner, Bauder, Sommer, Dettmers, Taub & Weiller, 1998). The patients were at least 6 months poststroke (mean chronicity = 6 years) and were able to extend at least 20 degrees at the wrist and 10 degrees at the fingers on their impaired extremity prior to therapy. Patients received 14 days of CI Therapy; they were trained to use their paretic arm for 6 hours each day in the clinic and wore a sling on their contralateral arm in the clinic and at home for 90% of waking hours. The amount of arm use at home was measured using the Motor Activity Log (MAL), which is a semi-structured interview that tracks arm use on 20 activities of daily living (Taub & Uswatte, in press; Taub, Miller, Novack, Cook, Fleming, Nepomuceno, Connell, & Crago, 1993). Cortical reorganization was measured by mapping the cortical output area of the abductor pollicis (APB) muscle using TMS with a figure-of-8 coil (Magstim, Dyfed, UK). Several parameters of brain activity were calculated. Data is presented here for the cortical representation area size of the APB muscle, defined as the number of scalp positions from which stimulation (10% above the individual motor threshold) produced at least 1 motor evoked potential (MEP) > 50 ÁV in a series of 5 stimulations. The ratio of affected to unaffected hemisphere representation area was used as the dependent measure because this ratio had been found to be a more stable measure of cortical representation than the absolute values for the individual hemispheres (Cicinelli, Traversa, & Rossini, 1997).
Study 2: Neuroelectric Source Imaging
Current source density analysis of the steady state electroencephalographic (EEG) motor potential was used to measure brain activity related to arm movement in 4 patients with a chronic upper extremity hemiparesis before, immediately after, and 3 months after CI Therapy (Kopp, Kunkel, Muehlnickel, Villringer, Taub & Flor, submitted). The patients were at least 1 year poststroke (chronicity range = 4-15 years) and were able to extend at least 20 degrees at the wrist and 10 degrees at the fingers on their impaired extremity. As in the first study, patients received 14 days of CI Therapy. The amount of arm use at home was measured using the MAL. Cortical reorganization was measured by dipole modeling of the mean source of steady-state movement-related cortical potentials (Gerloff, Toro, Uenishi, Cohen, Leocani, & Hallet, Electroenceph. Clin. Neurophysiol., pp. 106-113, 1997). The EEG was recorded from an array of 50 scalp Zn-electrodes while patients flexed/extended the digits of either their affected or unaffected hands. Following preprocessing of the data by a principle component analysis (PCA), a fixed dipole model was used for analysis of the first component of the PCA, and a single dipole was reconstructed for the time range from 20 to 400 ms after movement onset.
Results
Study 1: Transcranial Magnetic Stimulation
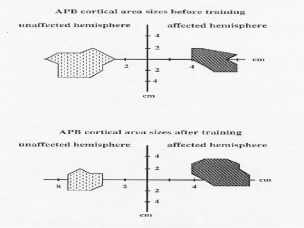
CI Therapy produced a large and significant increase in the patients' amount of arm use in the real world environment over the 2-week treatment period, going from a 2.3 (2 = sometimes used affected arm, but mostly used other arm) before treatment to 3.8 (4 = almost as much use as before the stroke) after treatment, p < .001. Over the same period, it was found that the cortical region from which EMG responses of the APB muscle could be elicited by TMS more than doubled, p < .01. Figure 1 illustrates this large change in the cortical area controlling affected arm movement associated with the increase in arm use produced by CI Therapy.
Fig. 1. Changes in cortical motor area sizes in the damaged hemisphere of a single subject eliciting responses in abductor pollicis brevis (APB) muscle before and after CI Therapy.
Study 2: Neuroelectric Source Imaging
As in Study 1, Kopp and associates found that CI Therapy produced a large and significant increase in the patients' amount of arm use in the home over the 2-week treatment period, going from a 2.0 (sometimes used affected arm, but mostly used other arm) before treatment to 4.7 (close to as much amount of use as before the stroke) after treatment, p < .001. Before and immediately after treatment, the mean source locations of motor potentials for movement of the affected arm were located in the damaged hemisphere anterior to the motor cortex. However, 3 months after treatment, the mean source locations for the affected arm had shifted from the stroke-damaged hemisphere to the undamaged hemisphere for all 4 patients and clustered in all three dimensions with the sources, presumably in primary motor cortex, that were activated by movements of the unaffected hand in the stroke patients and of the corresponding hand in 20 healthy control subjects. The fact that this effect was not in evidence immediately after treatment suggests that the shift in the mean source of affected arm motor potentials was due to the sustained increase in affected arm use over the 3-month follow-up period subsequent to treatment. Figure 2 illustrates the large shift in the mean source of motor potentials for movement of the affected arm from the hemisphere contralateral to the affected arm to the ipsilateral motor cortex, which normally controls movements of the contralateral (unaffected) arm.
Fig.2. Mean source locations superimposed on coronal and sagittal MRI scans of one patient. The source locations are for the left and right (affected) hand movements of subjects with chronic stroke (N = 4) before, immediately after, and 3 months following CI Therapy and for left and right hand movements of healthy subjects (N = 20).
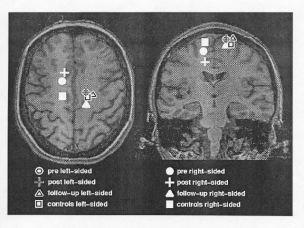 Figure 2
Figure 2
Discussion and Conclusion
The treatment data from the two studies presented here replicate the finding of Taub and co-workers (Taub et al., 1993; Taub, Pidikiti, DeLuca, & Crago, 1996; Taub & Uswatte, in press) that CI Therapy produces large increases in the amount of real world arm use of persons with a stroke-related paresis of an upper extremity. The two studies present the new finding that the increase in affected arm use produced by CI Therapy is associated with cortical reorganization of the areas controlling arm movement. Liepert and co-workers found that the cortical region from which EMG responses of a hand muscle could be elicited by TMS was more than doubled, while Kopp and associates found that 3 months after treatment the motor cortex ipsilateral to the affected arm, which normally controls movements of the contralateral arm, had been recruited to generate movements of the affected extremity.
These findings suggest that CI Therapy produces a permanent increase in arm use by two mechanisms. First, CI Therapy changes the contingencies of reinforcement (provides opportunities for reinforcement of use of the affected arm by constraining the unaffected arm) so that the learned nonuse of the stroke-affected arm conditioned in the acute and early subacute periods is counter-conditioned or lifted (For a detailed discussion of this mechanism see Taub, 1980 and Taub & Uswatte, in press). Second, the consequent increase in use, involving sustained and repeated practice of functional arm movements, induces expansion of the contralateral cortical area controlling movement of the affected arm and recruitment of new ipsilateral areas. This use-dependent cortical reorganization may serve as the neural basis for the permanent increase in use of the affected arm that results from CI Therapy. Moreover, to the best of our knowledge, these recent studies are the first to demonstrate an alteration in brain structure or function associated with therapy-induced rehabilitation of movement after CNS damage in humans.
Supported by grants from the Deutsche Forschungsgemeinschaft, Grant #HD34273 from the National Institutes of Health, Grants B93-629AP and B95-975R from the Rehabilitation Research and Development Service, U.S. Department of Veterans Affairs, and Grant #94-172 from the Retirement Research Foundation.
References
- Liepert, J., Bauder, H., Sommer, M., Miltner, W.H.R., Dettmers, C., Taub, E., & Weiller, C.. (1998). Motor cortex plasticity during Constraint-Induced Movement Therapy in chronic stroke patients. Neuroscience Letters, 250, 5-8.
- Kopp, B., Kunkel, A., Muehlnickel, W., Villringer, K., Taub, E.,& Flor, H. (1998). Plasticity in the motor system correlated with therapy-induced improvement of movement in human stroke patients. Manuscript submitted for publication.
- Taub, E. (1980). Somatosensory deafferentation research with monkeys: Implications for rehabilitation medicine. In L. P. Ince (Ed.), Behavioral psychology in rehabilitation medicine: Clinical applications (pp. 371-401). Baltimore, MD: Williams & Wilkins.
- Taub, E., Miller, N. E., Novack, T. A., Cook, E. W. III, Fleming, W. C., Nepomuceno, C. S. Connell, J. S., & Crago, J. E. (1993). Technique to improve chronic motor deficit after stroke. Archives of Physical Medicine and Rehabilitation, 74, 347-354.
- Taub, E., Pidikiti, R.D., DeLuca, S.C., & Crago, J.E. (1996). Effects of motor restriction of an unimpaired upper extremity and training on improving functional tasks and altering brain/behaviors. In J. Toole (Ed.), Imaging and neurologic rehabilitation (pp. 133-154). New York: Demos Publications.
- Taub, E., & Uswatte, G. (in press). A new approach to treatment and measurement in physical rehabilitation: Constraint-Induced (CI) Movement Therapy. In R. G. Frank and T. R. Elliott (Eds.) Handbook of rehabilitation psychology. Washington, DC: American Psychological Association.
| Discussion Board | Previous Page | Your Symposium |