Invited Symposium: Behaviour-Induced Neural Events after Brain Injury
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
It has become increasingly clear that the brain of the adult mammal is plastic. Research has demonstrated morphological changes in the central nervous system resulting from factors such as environmental complexity, learning, and brain injury [1-7]. Structural changes in cortical neurons, such as enhanced dendritic arborization and increased synaptic connectivity, have been shown in the hippocampus [2,8], motor cortex [9], and cerebellum [3]. Recently, it has been shown that damage to the adult brain leads to morphological changes in connected cortical areas remote from the primary site of injured tissue [10]. These neuronal changes may play a role in two distinct processes: degenerative effects resulting from the insult and plastic changes vital to adaptation to the insult.
In response to unilateral electrolytic lesions to the forelimb representational area (FLA) of the sensorimotor cortex (SMC), a variety of reactive effects have been seen in the contralateral homotopic cortex, including transient increases in cortical thickness, dendritic branching, and synapse number [4,5,7,11]. These morphological changes have been demonstrated to correlate with behavioral changes, such that the forelimb-use asymmetry that results from unilateral damage is greatest preceding increased arborization and decreases to pre-lesion levels at about the time of onset of dendritic branching (30-120 days post-lesion) [5]. Further, it has been demonstrated that the extent to which morphological changes occur in the cortex contralateral to the site of damage is dependent upon both the damage itself and the use of the non-damaged hemisphere. Unilaterally lesioned rats allowed to use only their non-damaged forelimb (ipsilateral to the damaged FLA) during the recovery period had larger increases in dendritic arborization in the undamaged cortex than those that used only their damaged limb or those that had no lesion but were forced to over use one limb [6]. Thus, both the injury and the resultant behavioral changes are necessary to enhance morphological changes. It may be that the morphological changes found in the contralateral FLA result from forelimb asymmetrical use that involves learning.
It is therefore evident that damage to the SMC can have large morphological effects upon related cortical areas, and that these effects may be enhanced through behavioral skill training. A logical next step in continuing research on the effects of, and recovery from, SMC damage is to investigate other regions of the brain relevant to motor skill and behavior, specifically the cerebellum. Like the FLA of the SMC, the forelimb representational area of the cerebellum, the paramedian lobule, is plastic, and various morphological alterations have been detected following both motor exercise and motor skill learning [3]. As it is assumed that a certain degree of new skill learning is involved in the recovery of function following damage to the SMC, it is important that the role of the paramedian lobule of the cerebellum in recovery be investigated. This study was designed to address the following two questions. First, does the paramedian lobule show compensatory responses to SMC damage? Second, does behavioral experience effect the strength of these changes?
Materials and Methods
30 adult male Long Evans hooded rats, age 2-3 months, were divided randomly into two surgical treatment groups: a lesion group, receiving bilateral electrolytic lesions to the FLA of the SMC, and a sham group. Prior to surgery, rats were anesthetized with a 65mg/kg intraperitoneal injection of sodium pentobarbital. For the lesion group, skull and dura were removed in both hemispheres above the forelimb representational area of the SMC (between 0.5 mm posterior and 1.5 mm anterior to bregma, and between 3.0 mm and 4.5 mm lateral to midline). An electrode was lowered to 1.7 mm below dura, and twelve 10s trains of DC current (1mA) were delivered at five equally spaced points on the horizontal trajectory. For rats in the sham group, identical surgical procedures were followed up to removal of the skull. All rats were allowed eight days of recovery before beginning behavioral training.
The rats were further divided into three treatment groups with an equal representation of lesion (n=5) and sham (n=5) animals in each group: an acrobatic condition (AC), motor condition (MC), and inactive condition (IC). Because two lesion animals did not survive the surgery, two treatment groups contained only 4 lesioned animals (AC & MC). Animals in all groups received 21 days of training. AC animals were trained on an obstacle course consisting of 10 complex tasks that required the acquisition of considerable motor skill to master. Obstacle sets were changed every seven days in increasing order of difficulty to assure that learning occurred throughout the entire training period. Rats traversed the course 5 consecutive times per day. Errors, defined as any misplacement of a forelimb, were counted and the time per task was recorded. MC rats ran 25 laps on a flat running alleyway, therefore receiving the same amount of motor exercise as the AC rats but no skill learning. IC rats received no significant motor exercise or skill learning. They were handled daily for 5 minutes to control for effects of stress and human interaction. Figure 1 depicts the three training conditions.
 Figure 1.
Figure 1.After day 21 of training, the rats were overdosed with sodium pentobarbital and perfused with 200 ml of (0.1M) phosphate buffer and 400 ml of 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde overnight then cryoprotected in a 30% sucrose solution for at least 24 hrs. The brains were blocked between the cerebellum and cerebrum, and the cerebelli and motor cortex were sliced at 52 µ thickness, sagitally and coronally respectively. Histological verification of the lesion site was performed on the motor cortex using Prussian Blue. Representative cerebellar slices, spanning the entire extent of the paramedian lobule, were selected and a standard immunohistochemical procedure using an antibody against the AMPA glutamate receptor was performed (anti-GluR 2; Chemicon).
Sections were washed in phosphate buffered saline (PBS) and soaked in 0.3% hydrogen peroxide for 60 minutes at room temperature to catalyze the endogenous peroxidases. Tissue sections were then washed in PBS and 2% horse serum (HS) before being blocked overnight (10% HS and 0.5% Triton-X in PBS) at 4 °C. On the following day, sections were washed with PBS then 2% HS, and placed in primary antibody solutions (10% HS, 0.5% Triton-X, and a 1:500 dilution of primary) overnight at 4 °C. On the third day of the procedure, sections were washed (2% HS in PBS) and placed in a secondary antibody solution (10% HS and 0.5% biotinylated, horse anti-mouse IgG secondary antibody, in PBS) for 90 minutes at room temperature. Next, tissue sections were washed in PBS and 2% HS, before being placed in avidin-biotin complex solution for 60 minutes at room temperature. Sections were then washed in PBS and TRIS buffer. Tissue was reacted in a 3,3'-diaminobenzidine (DAB) solution (100mg DAB, 1.39g nickel ammonium sulfate and 6.67ml hydrogen peroxide in 200ml TRIS buffer) for approximately 10 minutes. Finally, sections were washed in PBS 5 times before being mounted onto gelled slides. After drying overnight, slides were cleared and coverslipped.
Cerebellar images were then entered into the MCID densitometry system for analysis. Relative optical density (ROD) of AMPA receptor labeling was sampled from multiple points in the molecular and granular layers of the paramedian lobule, and signal to noise ratios (SNR) were used to compute percent change in density from white matter (i.e. [(SNRmol - SNRwhite matter) / SNRwhite matter] * 100). Data were then analyzed on a mainframe using SAS.
Results
Behavioral Data:
Figure 2 shows AC animals' mean completion time for one circuit of the obstacle course, separated by the three obstacle sets. Planned comparisons using the Least Significant Difference test (alpha=.05) indicated that lesion animals were significantly slower than shams.
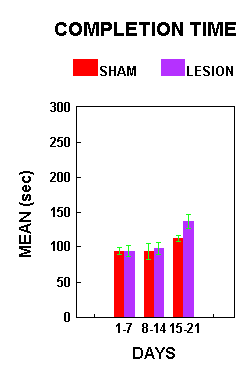 Figure 2.
Figure 2.Figure 3 illustrates the mean number of errors made by the AC animals per training circuit. There was a significant effect of treatment (F(1,7)=17.99, p=.0038). Lesioned animals made significantly more errors than sham animals. Planned comparisons (alpha=.05) indicated that lesion animals made significantly more errors than sham animals during both the first and third week of training.
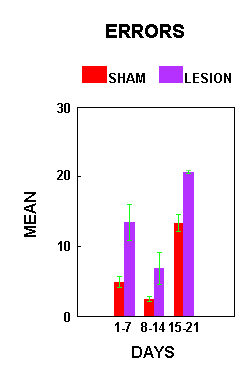 Figure 3.
Figure 3.Figure 4 shows the percent decrease in number of errors made by the AC animals on the acrobat course across the full 21 days of training. There was no significant difference between lesion and sham animals relative to the percent decrease in number of errors made (F(1,7)=0.02, p=0.8904).
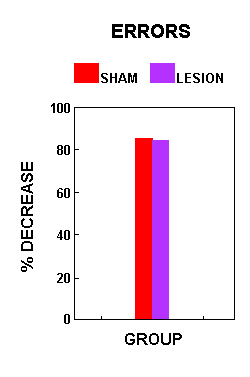 Figure 4.
Figure 4.Morphological Data:
Figure 5 shows a representative section from one of the lesioned animals. In all cases, the extent of motor cortex damage was substantial.
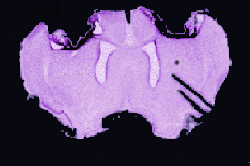 Figure 5.
Figure 5.Figure 6 illustrates the percent change in relative optical density of the staining in the three training groups, collapsed across surgical treatment. There were no significant group differences in ROD (F(2,22)=1.09, p=0.3524). Planned comparisons (alpha=.05) indicated that MC animals showed a significantly larger increase in immunoreactivity than both the AC and IC treatment groups. The percent change in ROD was not significantly different between the AC and IC groups.
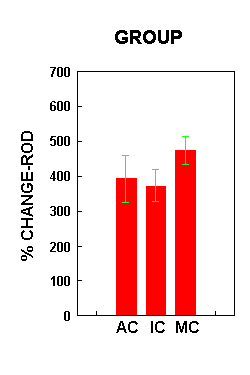 Figure 6.
Figure 6.Figure 7 illustrates the percent change in ROD across the three training groups relative to surgical condition. There was a significant interaction effect between surgical treatment and group (F(2,22)=4.28, p=0.027). Planned comparisons (alpha=.05) showed a significant difference in percent change in ROD between the lesion and sham animals within each treatment group. In both the AC and MC group, the lesion animals demonstrated a significantly larger increase in the density of receptor staining than the sham animals. In the IC group, lesion animals showed a significantly lower intensity of AMPA receptor staining than shams.
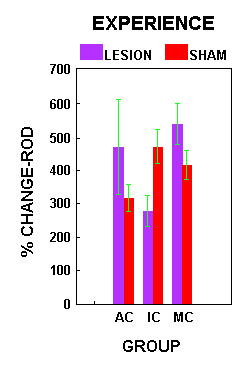 Figure 7.
Figure 7.Discussion and Conclusion
The data indicated that motor cortex damage led to impaired motor performance on the acrobatic course. However, both the lesion and sham animals showed a decrease in the number of errors across time, suggesting that both groups acquired new motor skills. The ability to acquire new motor skills was apparently preserved in the lesioned animals, as the decrease in number of errors was similar for both the lesion and sham groups. We have found in our lab that the cerebellum shows an increase in AMPA receptor density following motor skill learning (unpublished). Thus, we believe that the cerebellum may contribute to this preservation of motor skill learning.
The morphological data support this hypothesis. Lesioned animals in both the AC and MC group showed increased AMPA receptor labeling in the cerebellum. This increase was found only in the two motor conditions, emphasizing the importance of motor skill learning and motor exercise for enhancing adaptive morphological changes. The inactive rats (IC) demonstrated the opposite effect, with higher receptor densities in the sham animals. It is hypothesized that this loss of receptor staining in lesioned ICs may reflect degenerative changes associated with deafferentation.
The conclusions from this study are threefold. First, there is obvious behavioral impairment following damage to the sensorimotor cortex, though the ability to learn motor skills appears to be spared. Second, given that the cerebellum, a motor region of the brain, demonstrated morphological changes in response to damage of the sensorimotor cortex, cerebellar compensatory changes may be responsible for the sparing of skill acquisition abilities. Finally, morphological changes are enhanced only under conditions in which the animal is actively engaging in motor behavior.
References
- Comery, T.A., Shah, R. & Greenough, W.T. (1995). Differential rearing alters spine density on medium sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiology of Learning and Memory, 63, 217-219.
- Greenough, W.T., Larson, J.R. & Withers, G.S. (1985). Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behavioral and Neural Biology, 44, 301-314.
- Black, J.E., Isaacs, K. R., Anderson, B.J., Alcantara, A.A. & Greenough, W.T. (1990). Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Science, 87, 5568-5572.
- Jones, T.A. & Schallert, T. (1989). Sensorimotor cortex lesions: time-dependent anatomical changes specific to the contralateral homotopic hemisphere. Society of Neuroscience Abstracts, 15, 1223.
- Jones, T.A. & Schallert, T. (1992). Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Research, 581, 156-160.
- Jones, T.A. & Schallert, T. (1994). Use-dependent growth of pyramidal neurons after neocortical damage. Journal of Neuroscience, 14, 2140-2152.
- Jones, T.A., Kleim, J.A. & Greenough, W.T. (1996). Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Research, 733, 142-148.
- Withers, G.S. & Greenough, W.T. (1989). Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia, 27, 61-69.
- Kleim, J.A., Lussnig, E., Schwarz, E.R., Comery, T.A. & Greenough, W.T. (1996). Synaptogenesis and FOS expression in the motor cortex of the adult rat after motor skill learning. Journal of Neuroscience, 16, 4529-4535.
- Castro-Alamancos, M.A. & Borrell, J. (1995). Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience, 68, 793-805.
- Kozlowski, D.A., James, D.C. & Schallert, T. (1995). Dendritic arbor versus spine density changes after unilateral injury to sensorimotor cortex: temporal pattern, use- dependency and relation to recovery of function. Neurotrauma Society Abstracts, 1995.
| Discussion Board | Previous Page | Your Symposium |