Invited Symposium: Perspectives on Behavioural Function of Dopamine in the Nucleus Accumbens
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Interactions between excitatory amino acid (EAA) and dopamine (DA) in the basal ganglia have been known for some time to contribute importantly to the generation of motor behaviors. In particular, the role played by ionotropic glutamate receptors (iGluRs) in such interactions and in the production of locomotion has received considerable attention. Recently, the growing importance of metabotropic glutamate receptors (mGluRs) in such behaviors as well as in various forms of plasticity has begun to emerge. The known coupling of the mGluR to second messenger systems and its demonstrated role in the long-term modulation of synaptic transmission make it a logical candidate not only for the generation of locomotion involving EAA-DA interactions but also for the induction and expression of locomotor plasticity involving these neurotransmitters. In the experiments described below, we investigated the contribution of mGluRs in the nucleus accumbens (NAcc) to locomotor behaviors induced by amphetamine (AMPH). The NAcc constitutes the major subcortical terminal field of DA perikarya located in the ventral tegmental area and receives extensive glutamatergic projections directly from the prefrontal cortex and limbic structures such as the hippocampal formation and amygdala. Ultrastructural studies of the NAcc have indicated that some of the terminals of the descending EAA projections from cortex and those of ascending DA mesencephalic projections not only come in close apposition to each other but form synaptic contacts with the same intrinsic NAcc neurons as well. Considering that these latter neurons project to motor output regions, such an anatomical arrangement provides the basis for possible behaviorally relevant interactions between DA and glutamate at the level of nerve terminals in the NAcc (see Vezina and Kim, 1999).
The Locomotion Produced by Activation of NAcc mGluRs is DA-Dependent
Recently, we and others (Attarian and Amalric, 1997; Kim and Vezina, 1997) reported that, in a manner consistent with the well documented contributions of the kainate, AMPA and NMDA subtypes of the iGluR to locomotor activation, activation of mGluRs in the NAcc also increased locomotor activity. Thus, in one experiment (Kim and Vezina, 1997), bilateral intracranial microinjections into the NAcc of the selective mGluR agonist, 1-aminocyclopentane-trans-1,3-dicarboxylic acid, (1S,3R)-ACPD, were made in the freely moving rat and locomotor activity was measured for 2 hours. Activation of mGluRs in the NAcc by different doses of (1S,3R)-ACPD (0.005, 0.05, 0.5 or 2.5 nmole/0.5microl/side) produced dose-dependent increases in locomotor activity and these effects were blocked by co-injections of the mGluR antagonist, (RS)-alpha-methyl-4-carboxyphenylglycine, (RS)-MCPG (2.5 nmole/side).
In order to evaluate the contribution of NAcc DA to these effects, the following two experiments were conducted. In the first, rats in different groups were administered bilateral microinjections into the NAcc of either saline, (1S,3R)-ACPD (0.5 nmole/side), the DA receptor antagonist fluphenazine (2.0 or 9.8 nmole/side) or a cocktail of (1S,3R)-ACPD and one of the two doses of fluphenazine. As can be seen in Fig. 1, the locomotor activating effects of (1S,3R)-ACPD in the NAcc were completely blocked when this agonist was co-injected with a DA receptor antagonist.
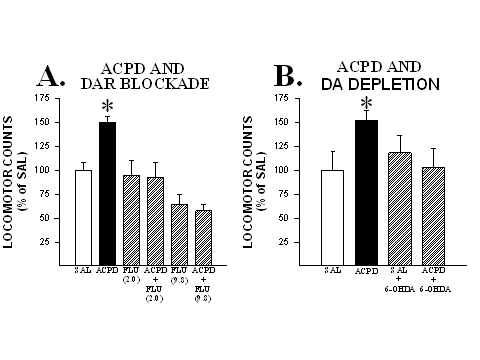 Fig. 1: DA is necessary for the generation of locomotion by mGluR activation in the NAcc. A. Co-injecting the DA receptor antagonist fluphenazine (2.0 or 9.8 nmole/side) with (1S,3R)-ACPD into the NAcc blocked the locomotor activity normally produced by this mGluR agonist. B. Depleting DA in the NAcc with 6-OHDA (42-99% depletions compared to control values) prevented the generation of locomotion by infusions of (1S,3R)-ACPD into this site. Locomotor data are shown as group mean (+SEM) percent of SAL observed in one hour of testing. n=6-10/group. Asterisks denote significant differences from SAL at the 0.05 level. Adapted from Kim and Vezina (1997) and Meeker et al. (1998).
Fig. 1: DA is necessary for the generation of locomotion by mGluR activation in the NAcc. A. Co-injecting the DA receptor antagonist fluphenazine (2.0 or 9.8 nmole/side) with (1S,3R)-ACPD into the NAcc blocked the locomotor activity normally produced by this mGluR agonist. B. Depleting DA in the NAcc with 6-OHDA (42-99% depletions compared to control values) prevented the generation of locomotion by infusions of (1S,3R)-ACPD into this site. Locomotor data are shown as group mean (+SEM) percent of SAL observed in one hour of testing. n=6-10/group. Asterisks denote significant differences from SAL at the 0.05 level. Adapted from Kim and Vezina (1997) and Meeker et al. (1998).
In the second (Meeker et al., 1998), different groups of rats were pretreated with bilateral microinjections of either 6-OHDA or its vehicle into the NAcc and, on separate tests starting 10 days later, were tested for locomotion following microinjections (into the same site) of saline and (1S,3R)-ACPD (0.5 nmole/side).DA levels at the microinjection sites were significantly depleted in 6-OHDA-treated animals (42-99% depletions compared to control values obtained in vehicle treated rats). As can also be seen in Fig. 1, in contrast to the expected increased locomotion observed in non-lesioned rats, rats pretreated with 6-OHDA showed no increase in locomotor activity in response to the intra-NAcc infusions of (1S,3R)-ACPD.
The findings of these two latter experiments indicate, therefore, that in a manner resembling AMPH, enhanced locomotion produced by NAcc mGluR activation is dependent on intact DA neurotransmission in this site.
NAcc mGluRs Contribute to AMPH-Induced Locomotion
Given the evidence presented above for the existence of functional interactions between mGluRs and DA terminals in the NAcc and the known contribution of NAcc iGluRs to AMPH-induced locomotion, the following experiments investigated the contribution of this receptor to the locomotor activating effects produced by acute injections of AMPH into this site (Kim and Vezina, 1998a).
In a first experiment, rats received microinjections into the NAcc of (RS)-MCPG (25 nmole/side) either alone or with AMPH (6.8 nmole/side) and their locomotion was measured. As can be seen in Fig. 2, when injected alone into the NAcc, this dose of (RS)-MCPG produced no locomotor effects. But when injected with AMPH into the NAcc., this mGluR antagonist completely blocked this drugís locomotor activating effects. This same dose of (RS)-MCPG was also found to block the locomotor effects of the D1 DA receptor agonist apomorphine (32.9 nmole/side) in the NAcc (Kim and Vezina, 1998a), suggesting that the contribution of mGluRs to AMPH-induced locomotion may be mediated by mGluRs expressed by NAcc cells postsynaptic to DA neuron terminals.
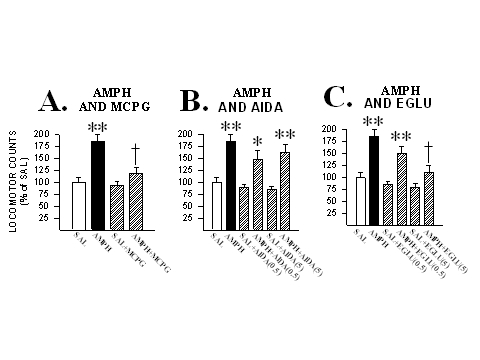 Fig. 2: mGluRs in the NAcc contribute to AMPH-induced locomotion. A. Co-injecting the mGluR antagonist (RS)-MCPG (25 nmole/side) with AMPH into the NAcc blocked the locomotor activity normally produced by this drug. B. Co-injecting the group I mGluR specific antagonist AIDA (0.5 and 5 nmole/side) with AMPH into the NAcc spared this drugís locomotor activating effects. C. Co-injecting the group II mGluR specific antagonist EGLU (0.5 and 5 nmole/side) with AMPH into the NAcc dose-dependently blocked the locomotor activity normally produced by this drug. Locomotor data are shown as group mean (+SEM) percent of SAL observed in one hour of testing. n=5-14/group. Asterisks denote significant differences from SAL; one, at the 0.05 and two, at the 0.01 level. Daggers denote significant differences from AMPH at the 0.05 level. Adapted from Kim and Vezina (1998a) and Kim et al. (1998).
Fig. 2: mGluRs in the NAcc contribute to AMPH-induced locomotion. A. Co-injecting the mGluR antagonist (RS)-MCPG (25 nmole/side) with AMPH into the NAcc blocked the locomotor activity normally produced by this drug. B. Co-injecting the group I mGluR specific antagonist AIDA (0.5 and 5 nmole/side) with AMPH into the NAcc spared this drugís locomotor activating effects. C. Co-injecting the group II mGluR specific antagonist EGLU (0.5 and 5 nmole/side) with AMPH into the NAcc dose-dependently blocked the locomotor activity normally produced by this drug. Locomotor data are shown as group mean (+SEM) percent of SAL observed in one hour of testing. n=5-14/group. Asterisks denote significant differences from SAL; one, at the 0.05 and two, at the 0.01 level. Daggers denote significant differences from AMPH at the 0.05 level. Adapted from Kim and Vezina (1998a) and Kim et al. (1998).
To further investigate this effect, a second experiment assessed the effects on NAcc-AMPH-induced locomotion of the goup I specific mGluR antagonist (RS)-1-aminoindan-1,5-dicarboxylic acid, AIDA (0.5-5.0 nmole/side), and the group II specific mGluR antagonist (2S)-alpha-ethylglutamic acid, EGLU (0.5-5.0 nmole/side). As also shown in Fig. 2, none of the doses of AIDA and EGLU tested, when injected alone into the NAcc, produced effects on locomotion that differed significantly from those produced by saline. However, when co-injected with AMPH (6.8 nmole/side), the higher dose of EGLU, but not AIDA, completely blocked AMPH-induced locomotion. These results, in a manner consistent with those of others on AMPH-induced increases in extracellular levels of DA in the NAcc (Hu et al., 1998), suggest that group II but not group I mGluRs contribute importantly to NAcc-AMPH-induced locomotion.
NAcc mGluRs and the Expression of Locomotor Sensitization by AMPH
In addition to the role for mGluRs in the generation of locomotor activity outlined above, there is evidence that this receptor might also contribute importantly to the expression of the sensitization of locomotor activity by psychomotor stimulant drugs. Given the importance of the NAcc for the expression of locomotor sensitization by drugs like AMPH (see Kalivas and Stewart, 1991), the following experiment investigated the potential role of mGluRs in this site to this effect (Kim and Vezina, 1998b).
Rats were administered four injections of saline or AMPH (1.0 mg/kg, IP), one injection every third day. Two weeks after the last injection, the animals were challenged with either saline, (1S,3R)-ACPD (0.5 nmole/side) or (RS)-MCPG (2.5 nmole/side) into the NAcc. Interestingly, (1S,3R)-ACPD-induced locomotion remained unaffected following AMPH pre-exposure. However, as can be seen in Fig. 3, (RS)-MCPG which had no effect on locomotion when given to saline pre-exposed rats, now produced significant increases in locomotion in AMPH pre-exposed rats. These findings indicate that glutamatergic neurotransmission mediated by mGluRs in the NAcc is altered by repeated systemic injections of AMPH and may thus contribute importantly to the expression of locomotor sensitization by this psychomotor stimulant.
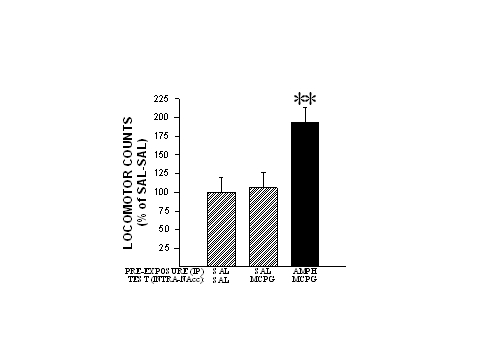 Fig. 3: The mGluR antagonist (RS)-MCPG produces increased locomotor activity when injected into the NAcc of AMPH pre-exposed rats. Locomotor data are shown as group mean (+SEM) percent of SAL-SAL observed in one hour of testing. (RS)-MCPG was injected into the NAcc at a dose 2.5 nmole/side. n=6-11/group. Asterisks denote significant differences from SAL-SAL at the 0.01 level. Adapted from Kim and Vezina (1998b).
Fig. 3: The mGluR antagonist (RS)-MCPG produces increased locomotor activity when injected into the NAcc of AMPH pre-exposed rats. Locomotor data are shown as group mean (+SEM) percent of SAL-SAL observed in one hour of testing. (RS)-MCPG was injected into the NAcc at a dose 2.5 nmole/side. n=6-11/group. Asterisks denote significant differences from SAL-SAL at the 0.01 level. Adapted from Kim and Vezina (1998b).
While not precluding the need for an intact dopaminergic afferentation of the NAcc, these results, considered together with those of other recent studies, suggest that long-term alterations in DA neurotransmission may not be directly involved in the above effects. Rather, they may be more associated with long-term changes in glutamatergic neurotransmission. The effects of (RS)-MCPG described above may, for example, be related to this antagonistís blockade of upregulated presynaptic mGluRs serving as autoreceptors on EAA afferents. The resulting failure of these autoreceptors to fulfill their autoinhibitory functions would lead to increased extracellular levels of glutamate that would activate glutamate receptors (concurrently with the activation by DA of DA receptors) expressed postsynaptically by intrinsic NAcc cells, thus leading to the generation of enhanced levels of locomotor activity (see Kim and Vezina, 1998b; Vezina and Kim, 1999).
Summary and References
The results reviewed above support a role for NAcc DA and mGluR interactions in the generation of locomotion and at least one form of plasticity: the sensitization of locomotor activity by psychomotor stimulant drugs. However, it is clear that detailed neuronal mechanisms remain to be determined. Their emergence will surely prove interesting as the availability of mGluR subtype selective ligands continues to grow and we continue to increase our knowledge of the precise location in basal ganglia and the identity of the processes expressing the different mGluR subtypes.
- Attarian, S, Amalric, M (1997) Microinfusion of the metabotropic glutamate receptor agonist (1S,3R)-ACPD into the nucleus accumbens induces dopamine-dependent locomotor activation in the rat. European Journal of Neuroscience, 9: 809-816.
- Hu, G et al. (1998) Stimulation of group II and III mGluRs inhibit dopamine release in nucleus accumbens of rat. Society for Neuroscience Abstracts, 24: 585.
- Kalivas, PW, Stewart, J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research Reviews, 16: 223-244.
- Kim, J-H, Vezina, P (1997) Activation of metabotropic glutamate receptors in the rat nucleus accumbens increases locomotor activity in a dopamine-dependent manner. The Journal of Pharmacology and Experimental Therapeutics, 283: 962-968.
- Kim, J-H, Vezina, P (1998a) Metabotropic glutamate receptors in the rat nucleus accumbens contribute to amphetamine-induced locomotion. The Journal of Pharmacology and Experimental Therapeutics, 284: 317-322.
- Kim, J-H, Vezina, P (1998b) The metabotropic glutamate receptor antagonist (RS)-MCPG produces hyperlocomotion in amphetamine pre-exposed rats. Neuropharmacology, 37: 189-197.
- Kim, J-H et al. (1998) Activation of group II metabotropic glutamate receptors is necessary for the generation of locomotion by amphetamine in the rat nucleus accumbens. Society for Neuroscience Abstracts, 24: 585.
- Meeker, D et al. (1998) Depletion of dopamine in the nucleus accumbens prevents the generation of locomotion by metabotropic glutamate receptor activation. Brain Research, in press.
- Vezina, P, Kim, J-H (1999) Metabotropic glutamate receptors and the generation of locomotor activity: Interactions with midbrain dopamine. Neuroscience and Biobehavioral Reviews, in press.
| Discussion Board | Previous Page | Your Symposium |