Invited Symposium: Perspectives on Behavioural Function of Dopamine in the Nucleus Accumbens
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction and General Methods
Single-unit data have shown that midbrain DA neurons respond to primary and conditioned rewards (Ljungberg, Apicella, and Schultz, 1992; Mirenowicz and Schultz, 1996). However, mesolimbic DA neurons also appear to respond to salient non-appetitive sensory stimuli (Horvitz, Stewart and Jacobs 1997) and to novelty (Ljungberg et al., 1992). The fact that VTA DA neurons respond to a category of stimuli beyond that of appetitive or reward events is consistent with the view that mesolimbic DA serves a behavioral function broader than the transmission of a reward signal. The data presented here demonstrate VTA DA responsiveness to salient sensory stimuli, regardless of whether the stimuli are salient by virtue of conditioned reward properties or by physical characteristics of the sensory event, such as intensity.
Cats were chronically implanted with a moveable microdrive, consisting of two outer cannulae which guided the dorsal/ventral movement of two inner cannulae oriented in the anterio-posterior plane. The bottom tips of the inner cannulae were aimed 5 mm above the VTA. A bundle of 7 flexible insulated nichrome microwires (32 or 64 um o.d.) was lowered through each inner cannula to a position approximately 1 mm above the VTA. Target coordinates for the VTA were A: 4.1-5.8, L: 0.8-1.5, H: (-3.0)-(-4.0). The microwire bundles were glued to the top of the inner cannulae , so that subsequent lowering of these cannulae resulted in ventral movement of the microwires. Microwires were soldered (along with EEG, EMG and EOG electrodes) to a 25-pin connector and the entire assembly was secured to the skull.
Single-unit recording was conducted in an isolated sound-attenuated recording chamber. Auditory stimuli (1 ms clicks) were delivered through a speaker mounted on the recording chamber wall, and visual stimuli were delivered through a clear plexiglas window. Neuronal activity was monitored on a computer sampling at 40,000 Hz, with the waveform of each action potential overlaid on a software oscilloscope (DataWave Technologies). The timestamp and waveform of each action potential was stored digitally on the computer.
Following 1 week of postsurgical recovery, animals were placed in the recording chamber and screened for the presence of isolated neuronal activity on one of the fourteen implanted microwires. Dopaminergic neurons were identified according to previously established criteria: long duration action potentials (> 2 ms), slow firing rate (2-8 spikes/s), and firing pattern characterized by single spikes, occasionally interspersed with bursts of decreasing amplitude (Bunney et al. 1973). Final neurochemical confirmation was obtained by administration of apomorphine (0.5 mg/kg), which produced at least 50% reductions in discharge rate.
Upon isolation of a single-unit, baseline activity was monitored for approximately 10 min to assess action potential waveform and firing characteristics of the cell prior to tests of sensory response to Auditory and visual stimuli; Auditory stimuli of varying intensities; and Auditory stimulus signaling reward delivery (see below).
At the conclusion of the experiments, animals were deeply anesthetized and a 20uA anodal direct current was passed through the electrodes from which units had been recorded. Animals were perfused with potassium ferrocyanide in formalin to produce a Prussian blue reaction. Following perfusions and removal of the brain, frozen sections were cut and mounted on slides. Slides were stained with neutral red to facilitate visualization of electrode tracks and blue-labelled recording sites.
VTA DA response to auditory and visual stimuli.
While recording VTA DA neuronal activity, the animal was presented a series of 50 or 100 auditory stimuli (1 ms, 73 dB) and the same number of visual stimuli (light flashes, 1.5 X 106 candle power, 1.9 X 107 lumens peak intensity) with a randomly varied interstimulus interval of 10-30 s.
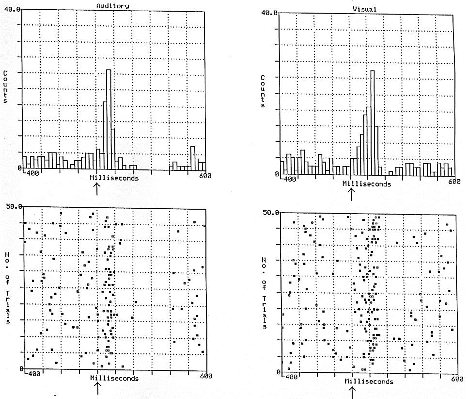 Fig. 1: Response of an individual ventral tegmental dopamine neuron to 50 presentations of a 1 ms
auditory click (left panel) and 50 presentations of a light flash (right panel) {from Horvitz, Stewart, and Jacobs, Brain
Research, 759, 251-258, 1997}
Fig. 1: Response of an individual ventral tegmental dopamine neuron to 50 presentations of a 1 ms
auditory click (left panel) and 50 presentations of a light flash (right panel) {from Horvitz, Stewart, and Jacobs, Brain
Research, 759, 251-258, 1997}
Auditory responses were observed in 13 of 15 ventral tegmental DA neurons (87%), visual responses were observed in 9 of 12 DA cells (75%). Auditory and visual responses appeared identical with the exception that the latency to peak visual response was longer than that of the auditory response (p<.01).
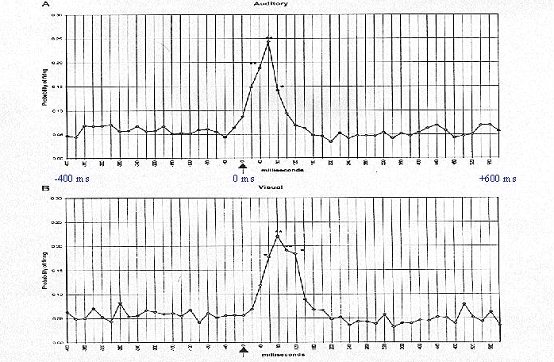
Fig. 2: Mean probability of VTA DA neuronal firing from 400 ms before to 600 ms after the delivery
of an auditory (top) or visual (bottom) stimulus (arrow). Probability estimates, at each time bin, were averaged across all
DA neurons tested for an auditory or visual response. Asterisks mark the poststimulus time bins for each neuronal
discharge for which discharge probability was significantly greater than that observed during the prestimulus baseline
(*P<0.05, **P<0.01). From Horvitz et al., 1997.
Varying stimulus intensity and conditioned properties of the stimulus
VTA DA response to auditory stimuli of varying intensities. Animals received 20 presentations of the 1 ms auditory event at each of the following intensities: 58, 66, 73, and 80 dB. The 4 different intensities were presented randomly (without replacement).
Increasing auditory intensity increased the likelihood of an action potential occurring between 0-150 ms after presentation of the auditory event (p < 0.01). Increased auditory intensities increased the likelihood of a dopamine burst response during a given trial (p< 0.01), but did not affect the duration of burst firing.
VTA DA response to a auditory stimulus signaling reward delivery.
A 1 ms click (73 dB) was followed, 1 sec later, by milk delivery, with a variable inter-trial interval of 15-60 sec.
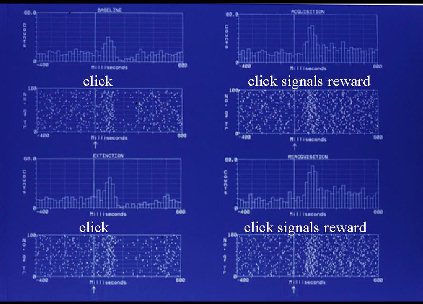
Fig. 3:The response of an individual VTA DA neuron is shown 400 ms before to 600 ms after presentation of a 1 ms click (arrow). The DA response to the auditory event is shown prior to classical click/milk pairings (top left, "click"), during CS/US conditioning
(top right, "click signals reward"), during extinction, i.e., click alone (bottom left, "click"), and during re-establishment of
the CS/US association (bottom right, "click signals reward").
As seen in the previous sensory tests, DA neurons were responsive to this fast rise-time, high-intensity auditory event even before it was paired with reward (top left). The DA response to the click was enhanced after it had come to predict reward delivery (top right). After conditioning, (bottom left) the DA response resembled that seen prior to conditioning. Reacquisition of the click/milk association (bottom right) reinstated the enhanced VTA DA neuronal response. After the click had become a conditioned signal for reward, it produced a VTA DA response that was elevated for several hundred ms beyond that seen when the same stimulus was not a predictor of reward. Only a small number (n=5) VTA DA neurons were subjected to this conditioned reward test, and conditioned elevations in burst duration were seen in 3 of the 5 cells, with the other cells unresponsive to the auditory event. It is of interest to note that while the DA neuronal response increased in duration when the click signaled imminent reward delivery, increases in auditory stimulus intensity elevated the likelihood of a DA neuronal burst response, but did not increase duration of the DA response.
Conclusion
DA neurons responded to nonconditioned, non-appetitive auditory and visual events. The DA response was elevated by a) increasing stimulus intensity and b) leaving intensity constant and pairing the event with an important outcome (milk delivery). The increase in stimulus intensity increased the likelihood of occurrence of a DA action potential in response to a given presentation of the sensory event, while pairing the event with reward also increased the duration of the neuronal response. One could argue that the VTA DA neuron transmits a signal of reward or conditioned reward by virtue of its sustained versus short duration response. However, it seems likely instead that the DA neuron responds to salient sensory events regardless of whether the salience is due to physical intensity or conditioned properties of the event. These DA elevations in response to salient events may serve to promote processing of afferent signals simultaneously arriving at the accumbens and other mesolimbic DA target structures.
References
Bunney, B.S., Walters, J.R., Roth, R.H. Aghajanian, G.K., 1973, Dopaminergic neurons: Effect of antipsychotic drugs and amphetamine on single cell activity, J. Pharmacol. Exp. Ther., 185, 560-571.
Horvitz, J.C., Stewart, T., and Jacobs, B.L., 1997, Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat, Brain Research, 759, 251-258.
Ljungberg, T., Apicella, P., and Schultz, W., 1992, Responses of monkey dopamine neurons during learning of behavioral reactions, J. Neurophysiol. , 67, 145-163.
Mirenowicz, J., and Schultz, W., 1996, Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli, nature, Nature, 379, 449-451.
| Discussion Board | Previous Page | Your Symposium |