Invited Symposium: Perspectives on Behavioural Function of Dopamine in the Nucleus Accumbens
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
INTRODUCTION
The focus of much of our research has been on understanding how the habit-forming properties of commonly abused drugs are related to their pharmacological action. The stimulants cocaine and amphetamine and the opiates heroin and morphine have in common the property of increasing central DA transmission. Experimental evidence suggests that the habit-forming property of these drugs is related to their ability to facilitate transmission in DA neurons located in the ventral tegmental area (VTA). This population of DA neurons innervates various limbic forebrain regions, notably the nucleus accumbens (NAcc) as well as several discrete cortical regions including the medial prefrontal cortex (PFC). It is believed also to be an important link in the brain circuitry that evolved to control feeding, copulation and other such species-typical behaviors.
Some of the more compelling evidence linking DA to the reinforcing action of rewards emerged from drug self-administration studies. In this model, animals are required to learn an arbitrary response, such as pressing a lever, to obtain a small intravenous drug injection. Under these conditions, stimulants and opiates act as potent rewards. As such, these drugs appear to reinforce learned behaviors in much the same way as an appetitive reward such as food would. Furthermore, it is generally believed that the reason these drugs are self-administered is because of their ability to increase brain DA levels. Implicit to this idea is the widely held assumption that increased DA transmission, especially in NAc, is the defining characteristic of rewards in general and of their positive reinforcing action. How valid is this assumption? While there is yet no definitive answer to this question, there is an emerging body of evidence that seems to contradict this and other key assumptions concerning mesocorticolimbic DA function. From our own studies, we have found little evidence that is consistent with the idea that increased NAcc DA transmission is a correlate of the reinforcing action of rewards in general, nor, for that matter, with the notion that the habit-forming property of drugs is a simple correlate of their pharmacological action on NAcc DA transmission. The present article summarizes some of the findings that led us to this conclusion.
The main focus here will be on the changes in NAcc DA transmission that accompany earned presentations of a food reward. These findings will be then be discussed in relation to prominent views on NAcc DA function and to a possible role of the PFC.
REWARD-RELEVANT CHANGES IN NAcc DA TRANSMISSION
Under the appropriate conditions, food can serve as a reward to reinforce operant responses and other learned associations. Evidence derived from behavioral studies indicates that increased DA transmission in NAcc, at some point, is necessary to generate behavioral responses to rewards. Exactly what causes this DA system to become more active is less clear. Increased NAcc DA transmission may be responsible for the behavioral activation elicited by the stimulus properties of food (e.g. sight and smell) and other distal cues that become associated with food. Alternatively, it may be that NAcc DA transmission increases in response to such positive, behavior-reinforcing outcomes as consuming earned food. While rewards can both incite and reinforce behavior, it is not clear which of these effects reflect increased DA transmission in NAcc. Feeding behavior had previously been reported to be associated with elevated DA levels in NAcc (Heffner et al 1980; Hernandez and Hoebel 1988; Radhakishun et al 1988; Yoshida et al 1992).
However, evidence from various other sources suggested that such increases in NAcc DA transmission do not occur as a consequence of food consumption (Blackburn et al 1986; Chance et al 1987; Blackburn et al 1989; Weatherford et al 1991; Blackburn et al 1992; McCullough and Salamone 1992; McCullough et al 1993; Phillips et al 1993; Salamone et al 1994). This conclusion also emerged from our in vivo electrochemical recording studies.
One objective of our research was to obtain detailed information on the changes in NAcc DA transmission that occur during the few minutes that precede and that follow the moment an animal earns a reward. Our working hypothesis was that the changes in synaptic DA transmission that occur just before and immediately after an earned reward would be the most significant for reinforcing learned behaviors. Voltammetry is well suited for this type of work because it allows fluctuations in extracellular DA levels to be monitored on a second-to-second basis. Voltammetry offers several additional advantages over other available techniques. The small carbon-fiber recording electrode is biologically inert, produces minimal tissue damage and provides excellent spatial resolution.
In addition, there is 8-12 day window during which the electrode’s sensitivity and selectivity for DA remains relatively stable. thus day-to-day differences in the acute effects of drugs, for instance, can be studied in the same animal. For the experiments described here, male rats each implanted with a monoamine-selective electrochemical probe in the NAcc were tested in a recording chamber equipped with a lever During testing, depression of the lever would trigger delivery of the condensed milk solution via a spout that protruded from the chamber wall and was connected by a length of polyethylene tubing to a syringe pump. The start of each session was signalled by illuminating the chamber light for 30 sec after which the spout was inserted into the chamber and the glass jar covering the lever was removed. Under the standard condition, each lever-press resulted in the delivery of 0.2 ml of condensed milk over 30 sec (flow rate=7 µl/sec); lever-presses during the period of milk delivery had no programmed consequences.
Each reinforced lever-press also caused the chamber light to be illuminated concurrently with the period of milk delivery. Animals were tested on consecutive daily 60-90 min sessions during which the pattern of electrochemical signal changes produced under different experimental conditions were examined. In general, the results of this study suggested that the DA projection to NAcc is not activated so much by gustatory stimuli as it is by cues that signal the availability of food. They also indicated that the main consequence of food consumption is a suppression of such anticipatory increases in NAcc DA transmission. This finding, more than any other, is at odds with the idea that increased DA transmission in NAcc is a central correlate of the behavioral reinforcing action of rewards. Insofar as the period of milk consumption is the critical requirement for positive reinforcement, it is a widely held assumption that this period would also be associated with increased DA release in NAcc. This assumption appears to be falsified by a number of findings.
Experience-dependent changes in NAcc DA. Throughout the first 3 days of testing, we observed small changes in electrochemical signal that were time-locked to each lever-press and to the 30 sec delivery of milk that followed. However, there were both within- and between-session differences in the magnitude and the direction of the electrochemical responses associated with each lever-press. As can be seen in Fig 1A, the initial few lever-presses of each session were followed by increases in signal. The most robust of these increases were observed on Day 1. Signals would start to rise 5-10 sec into the period of milk consumption and, upon termination of milk delivery, would either start decreasing slowly or continue to increase at a slower rate as the animals continued to lick the spout briefly before returning to the lever. In those animals that responded at regular intervals, the initial few bouts of milk consumption resulted in a stepwise elevation in the electrochemical signal.
As the session progressed, however, increments in signal gradually became smaller and tended to occur later during milk delivery until eventually little if any signal increase could be observed. If anything, decreases in signal were more typical of the changes associated with milk consumption during the latter period of the session. When increases were seen, these were small in comparison to those observed at the beginning of the session and usually occurred after the animal had finished consuming milk.
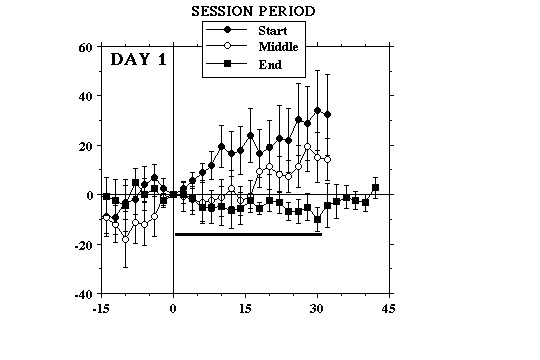 Fig. 1A
Fig. 1A
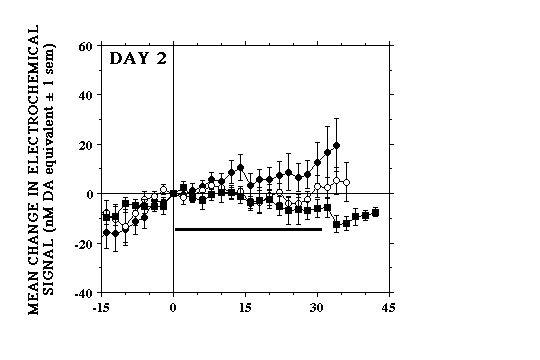 Fig. 1B
Fig. 1B
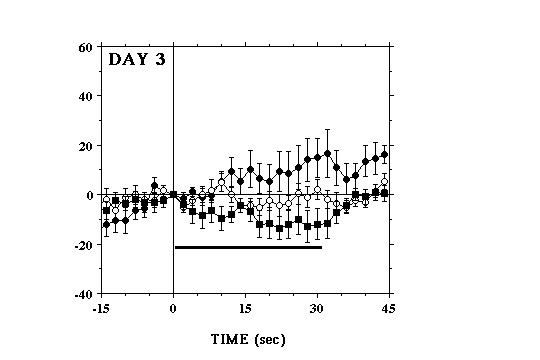 Fig. 1C
Fig. 1C
Similar within-session differences in the electrochemical responses to each lever-press were observed on the 2nd (Fig. 1B) and 3rd (Fig. 1C) test days. However, in comparison to Day 1 fewer of the initial bouts of milk consumption elicited signal increases and these were generally smaller and rose more slowly. Lever-presses that were followed by signal decreases also tended to be observed earlier in the session and, by the end of the 3rd test day, these were clearly more pronounced than at any time during the previous two test days. After as little a one day of testing, increases in signal were seen at the beginning of the session, before the animals were given access to the lever (Fig. 2).
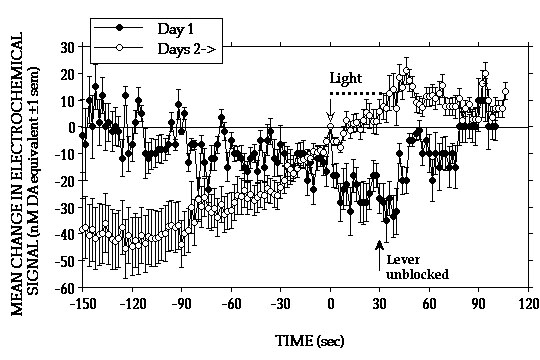 Fig. 2.
Fig. 2.
Such increases in signal were observed in all animals tested, although the magnitude varied from one day to another and from one animal to another. While increases in signal coincided with the 30 sec light cue that marked the start of the session, increases were also observed as early as 5 min prior to presentation of the light cue. During this period the syringe-pump was loaded with a fresh supply of condensed milk and the sounds associated with these preparations often caused animals to lick the wall area where the spout would be located and to paw at the jar covering the lever. These behaviors usually intensified and signal increases accelerated during presentation of the light cue.
Thus, on the 2nd and subsequent test days, the single largest increase in DA signal would often occur during the few minutes that preceded the start of the session. In contrast, the initial signal increases seen on Day 1 coincided with consumption of the first earned milk reward of the session. Our data indicate that the ability of food reward to activate NAcc DA transmission decreases as a function of training. A similar conclusion has been suggested on the basis of electrophysiological data showing that activation of DA cell firing associated with consumption of earned food disappears progressively as a function of the animals’ training (Ljungberg et al 1992). These authors have also found evidence indicating that, with training DA cells are increasingly activated by stimuli that are predictive of food presentation (Ljungberg et al 1992; Schultz et al 1993). Consistent with this, our data suggest that increases in NAcc DA elicited on Day 1 by the initial few bouts of milk consumption, rather than becoming smaller on subsequent days, were being shifted earlier in time, in response to stimuli that signalled the start of the session.
Reward-dependent changes in NAcc DA.
The decreases in NAcc DA transmission were closely related to, if not a direct consequence of consuming the food reward. Delaying delivery of earned milk caused a corresponding delay in the onset of DA signal decreases and the duration of these decreases was bound by the period of milk consumption (Fig 3).
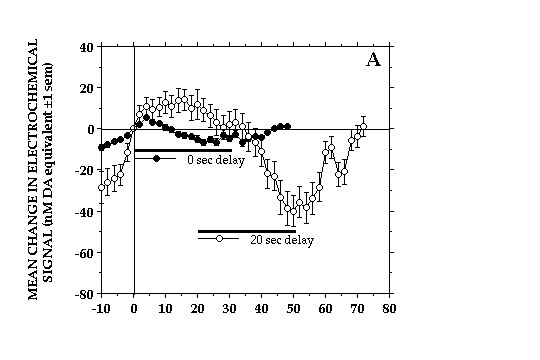 Fig. 3A
Fig. 3A
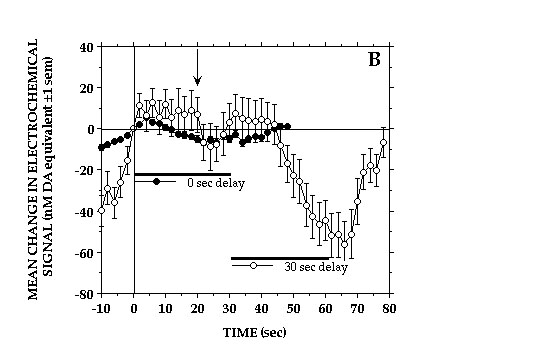 Fig. 3B
Fig. 3B
Orderly changes in DA signals were seen also as a result of varying the rate of milk delivery (Fig. 4A). Increasing the rate of delivery accelerated DA signal decreases associated with milk consumption. More important perhaps is that DA signals increases were seen when the standard delivery rate was halved. This finding plus the fact that DA signals also increased when earned milk was withheld is incompatible with the notion that NAcc DA transmission increases as a function of increasing reward magnitude. Rather, it would appear that changes in NAcc DA transmission reflect, at least in part, discrepancies between the rewards animals expect and the rewards they ultimately receive. That changes in NAcc DA transmission reflect changes in relative reward value is suggested also by the fact that only minor changes in DA signal accompanied consumption of milk delivered at 3-times the standard rate when this was the reward animals had been trained to expect (Fig. 4B).
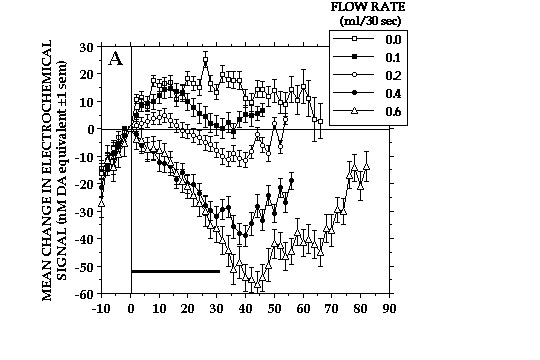 Fig. 4A
Fig. 4A
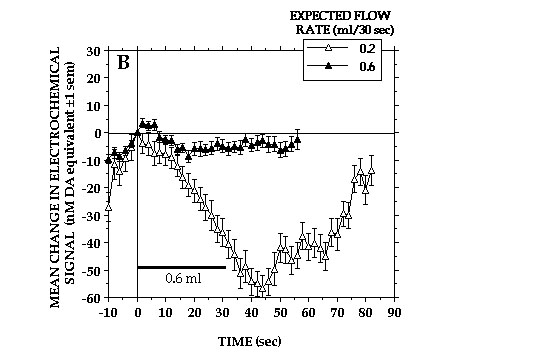 Fig. 4B
Fig. 4B
The important point here is that these animals received milk at the same rate that caused pronounced signal decreases in animals that had been trained to expect the standard reward.
Response-dependent changes in NAcc DA.
Changes in NAcc DA transmission were influenced also by the reinforcement schedule; that is, DA signal increases that preceded each reinforced lever-press and the signal decreases associated with the period of milk consumption that followed became more pronounced as a function of increasing response demands. That more pronounced increases in DA signals resulted from imposing a more demanding schedule would be expected since witholding earned milk also resulted in DA signal increases. This finding is in general agreement with electrophysiological data showing that responding on an FR20 or FR30 schedule causes ventral tegmental DA cells to increase their firing rate to the point of entering a burst firing mode (Nishino et al 1987).
Dopamine release increases monotonically with increasing cell firing, but will increase at faster rates as neurons start firing in bursts (Gonon and Buda 1985). These findings suggest that increased burst firing in meso-NAcc DA cells is responsible for the pronounced DA signal increases that accompanied unreinforced responses. Milk consumption under a partial reinforcement schedule resulted in DA signal decreases, the amplitude of which closely matched that of signal increases that preceeded reinforced response. This finding suggests that DA signal decreases associated with milk consumption depend not only on the magnitude of the reward, but also on the amount of work required to earn it. Since reward magnitude was held constant (0.2 ml/30 sec), milk consumption would have been associated with comparable signal decreases regardless of the reinforcement schedule had reward value been the only determining factor. This was clearly not the case here. While open to many interpretations, perhaps the simplest explanation for these data would be that of a contrast effect. Such an effect would occur as a result of a change in the value of the expected reward; that is, the contrast between two rewards of different values, in itself, diminishes the effectiveness of the lesser reward. In this case, the magnitude of the signal decrease would reflect the positive contrast between responses that went unrewarded and milk consumption that followed the reinforced response. The implicit suggestion here is that the magnitude of the positive shift in reward value was proportional to the number of unreinforced responses.
CONCLUSIONS
The findings described here are congruent with a body of evidence indicating that the DA projection to NAcc is responsible for mediating the behavior-activating effects of rewards and of other biologically-relevant conditions including a variety of aversive and stressful stimuli (Abercrombie et al 1989; Doherty and Gratton 1992, 1996). Our data indicate that food reinforcement can activate the DA projection to NAcc. However, this effect appears to be transient. One working hypothesis suggested by the present findings is that rewards produce their behavior reinforcing effects as a consequence of suppressing activation of meso-NAcc DA neurons by conditioned incentives. Exactly what mechanism would be responsible for exerting this inhibitory influence is open to speculation. The medial prefrontal cortex (PFC), however, is one possibility made increasingly attractive by evidence that meso-PFC DA neurons act indirectly, via corticofugal inputs to VTA and NAcc, to dampen concurrent increases in NAcc DA transmission.
| Discussion Board | Previous Page | Your Symposium |