Invited Symposium
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The juvenile spontaneously hypertensive rat (SHR), which is hyperactive, but not yet hypertensive, and is defective in sustained attention, features the main aspects of attention-deficit hyperactivity disorder (ADHD) in children (Sagvolden T., th is symposium; 1). A neuro-developmental disorder affecting about 1 % of the population, in a 4-to-1 ratio (Sergeant J.A., this symposium) . The evidence in the SHR model system indicates major alterations in the nigro-striatal and mesolimbic do pamine systems (Russell V., this symposium) innervating the caudate-putamen, the nucleus accumbens, and the prefrontal cortex, which are responsible for motor activity, attention, motivation and reward processes (Di Chiara G., this symposium) . Superfusi on experiments have demonstrated a "hypo-functioning" mesolimbic system in the SHR under basal conditions (see Russell V., this symposium), which is in accordance with radioligand binding studies showing an increased density of a particular sub type of receptor for the neurotransmitter dopamine 2. Therefore, at the level of the target there is an increased density of dopamine binding sites partially compensating for less dopamine being available at release sites. However, the increase d receptor density, which involves the D-1 but not the D-2 receptor type, is associated with less efficient signal transduction systems, such as the Ca2+/calmodulin dependent protein kinase ii) (CaMKII) 3 or transcriptional regulat ors of gene expression 4. It has been recently shown that this "defect" is restricted to the most rostral portions of the anterior basal forebrain only 5. CaMKIIwas chosen because is one of the major molecular devices in signal tran sduction. The transcription factor c-Fos was chosen because it is an immediate early gene (ieg) capable of modulating several target-genes through the orchestration involving members of different ieg family (see e.g. 5) . The use of multiple i maging probes, such as dopamine receptors, CaMKII and transcription factors have revealed a "segmental defect" in the anterior forebrain of SHR (Sadile A.G., this symposium).
This presentation focuses upon network changes which are monitored by the very same probes at sites distant from the defective segment and likely to be consequences of the hypothesized defect. To this aim, the sites of interest monitored by the imaging probes where the sub-cortical as well as the cortical areas connected to the frontal cortex, the caudate-putamen complex, the nucleus accumbens subterritories, pole, core and shell, and ventral pallidum. In fact the hypothesized segmental defect comprise s part of anterior portions of all structures within +2.70 and 1.70 with reference to bregma. In addition, cross correlative regional analyses were used to test the "cross-talk" among different sites within individual brains. Finally, experiment s were carried out to test the remodeling of neural networks by subchronic treatment with Methylphenidate (MP) which is known to block the re-uptake of biogenic amines, i.e. dopamine, serotonin, norepinephrine, epinephrine and histamine 6 (Sol anto-Gardner M., this symposium).
Materials and Methods
Animals: Juvenile (4-wk-old) male Spontaneously Hypertensive rats [SHR] and Normotensive Wistar-Kyoto controls (WKY) were obtained by a commercial breeder (Charles-River, Italy) . They were housed in standard cages during a two-wee k period for acclimatization and handling.
Procedures :Rats were either given a daily intraperitoneal injection of vehicle (controls) or methylphenidate (3 mg/kg) for 15 days. Rats were sacrificed either 1 day or 3 days after the last injection.
Forebrain removal and histology : for dopamine receptor binding and C.O. activity studies, the rats sacrificed 24 hours after the last MP or vehicle injection. Forebrains were quickly removed on ice, cooled at –30°C in isopentane, frozen in acetone-dry ice and stored at –80 degrees C. For CaMKII and c-FOS Icc, rats were deeply anesthetized by thiopental (60mg/kg) and perfused transcardially with physiological saline followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (PB, p H 7.3). Brains were postfixed in the same fixative for 2 h, washed in phosphate-buffered saline (PBS), soaked in 30 % sucrose for cryoprotection, frozen in isopentane on dry ice and stored at –80 deg C.
Receptor binding assays: The assays were carried out according to the procedure described earlier 2. A saturation analysis (maximal binding capacity and affinity) was performed using 7 concentrations of the radioactive DA antagonist [3H]-SCH 23390 (Spec. Act. 60-87 Ci/mM; 0.1 - 5.0 nM). D-1 excess unlabelled antagonist SCH 23390 was used, to control for non-specific binding. For D-2 receptor subfamily two competition studies were carried out using 4.2 nM 3 H-raclopride and 5 nM 3H-quinpirole with non-labeled spiperone (0.1 nM - 1 microM) and 7-OH-DPAT (0.1 nM - 10 microM) as non-labeled displacers. In both series of binding assays, ketanserin (1 microM) was added to resolve the DA and sero tonin components of the binding 7.
Autoradiography: The entire anterior forebrain was serially sectioned and randomly assigned to different experiments. The slides with tissue sections were incubated using optimized conditions and exposed to [3H]-sensitive films (Amers ham) for 18 days for SCH 23390, 8 weeks for raclopride and 3 months for quinpirole. Films were developed and optical densities (OD) of sections were analyzed with reference to co-exposed standard [3H]-microscale (calibration curve to convert OD to receptor concentration).
Immunocytochemistry: Fifty mm-thick cryostat coronal sections were collected in ice-cold PBS containing 0.1 % sodium azide. Sections were free-floating immunostained for the detection of the alpha-subunit of CaMKII 3 or c-FOS, as prev iously described 3,4. The primary antibody was a mouse monoclonal antibody towards the alpha-subunit of the CaMKII (Boehringer, Mannheim) or a rabbit polyclonal antibody against c-FOS. The c-FOS peptide was used to test for specificity. The d ilution of the antibody was 1:1000. The sections were exposed to diaminobenzidine (DAB, 100 mg in 200 ml buffer), and a Nickel-Cobalt intensification procedure was performed. To assess the specificity of labeling, control experiments were performed by inc ubating overnight the primary antibody with a ten-fold excess of peptide antigen in PBS. The sections were mounted on poly-L-lysine coated slides and coverslipped.
Quantitative C.O. histochemistry: twenty µm-thick coronal cryostat sections were serially taken across the brain. They were processed by the method of Gonzales-Lima 8.
Data acquisition and analysis: The autoradiograms were taken directly under a trans -illuminator, whereas icc and C.O. treated-sections were taken under a light microscope (Axioskop Zeiss) . In both cases images were acquired by a CCD camera (Ha mamatsu Photonics, Italia), and converted to a 640 x 480 pixel file by an acquisition board and a computer-assisted image analysis system (MCID-M4; Imaging Res. Inc, Canada). The recognition of forebrain structures was accomplished by inspection of Nissl- stained adjacent sections. For CaMKII and c-FOS immunoreactive elements, i.e. mainly somata and dendrites, were considered positive when they fell into the range between 0 and 140/160 (gray levels 0-255). For C.O. quantitative histochemistry, optical dens ities were transformed in units of enzyme specific activity by a standard curve using hearth tissue as a source of C.O. in presence of different amounts of brain tissue.
Data analysis and Statistics: For DA receptor binding studies saturation and competition analyses were carried out by conventional algorythmses. Maximal DA binding sites , proportional areas covered by CaMKII and c-FOS and C.O. specific activiti es full filled basic requirements for parametric analysis (homogeneity of variance, homoscedasticity, etc…). For each area data were submitted to three-way factorial ANOVAs line x treatment x brain side (as covariate) . Planned comparisons were made withi n area by two-tailed t-test for unpaired data, where as side differences were tested by t –test for paired data. Regional cross-correlation analyses were made by the Pearson product x moment method and the correlation coefficients were transformed in "z-s cores", which have a normal distribution. Planned comparisons were made using Student's t-test for unpaired data 9. The rejection level was set at 2 p < 0.05.
Results
1. REGIONAL CROSS-CORRELATION ANALYSIS FOR DOPAMINE RECEPTORS :
The degree of cross-talk was tested by correlating within each group individual levels of DA receptor binding sites among the sampled brain areas. In fact, as shown in Fig. 1, for D-1 and D-2 receptor families, In fact, average correlation score for D-1 binding sites was lower than that in WKY rats (WKY: 0.593 ± 0.170; SHR: -0.111 ± 0.201; df= 109; 2p < 0.01 by t-test for non paired data).
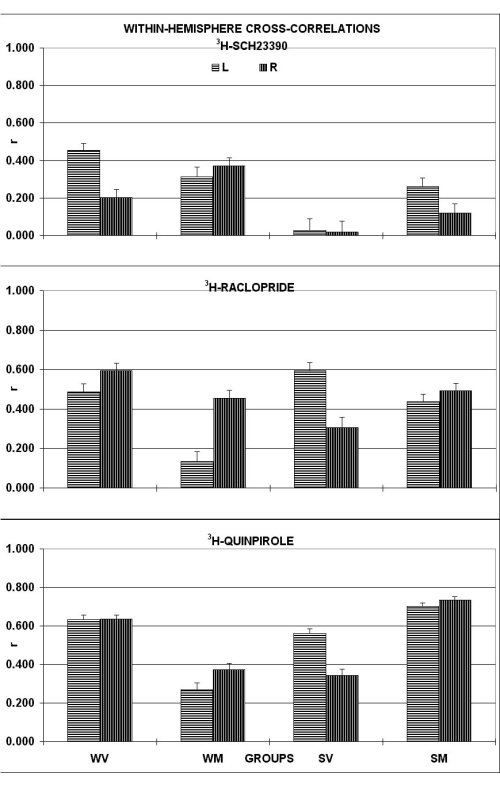 Fig. 1
Fig. 1
MP treatment had no significant effect in WKY and increased the synchronization in SHR (2p < 0.05). For D-2 family measured by 3H-raclopride binding, the degree of synchronization was similar in the two rat lines, but MP treatment desynchron ized the brains of WKY only (2p < 0.005). For D-2 receptor family, as measured by 3H-quinpirole binding, WKY and SHR were very similar in the cross-correlation score. However, MP treatment desynchronized receptor binding sites within the bra in of WKY (2p = 0.05) but not of SHR. The correlative profiles within each hemisphere indicate for D-1 family a higher cross-talk in the WKY within the left hemisphere, which was reduced by the MP treatment. In contrast, in the SHR there was no hem ispheric difference with a poor cross-talk within each hemisphere. Interestingly, MP treatment improved to a significant extent the cross-talk towards the level of non-treated WKY rats. For D-2 family, the correlative profiles obtained with raclopride and quinpirole were substantially similar. The cross-talk in the non-treated WKY rats was high on both sides with no left-to-right difference. In contrast, in the SHR the cross-talk was higher within the left than within the right hemisphere. MP treatment de monstrated a differential effect in the two groups. In fact, while it decreased the cross-talk within the left hemisphere in the WKY, it normalized the cross-talk in the SHR towards the level of the WKY rats with no left-to-right difference.
2. EXPRESSION OF CaMKII
Light microscope and computer-assisted high resolution image analysis (mcid-m2, imaging res. inc., canada) revealed: (i) A higher level of CaMKII-positive elements in both drug-treated groups. His effect pertained to all areas and was more pronounced in the SHR, but spared the nucleus accumbens pole and core, and the ventral pallidum in the WKY. (ii) In particular, in the SHR the drug reversed the reduced expression seen in the ventral pallidum and the cingulate cortex of vehicle-treated c ontrols. (iii) The beneficial effect of methylphenidate faded out within three days from the last injection (data not shown).
Within the amygdala complex, as shown in figure 2, under basal conditions there was not significant differences in the CamKII-positive elements.
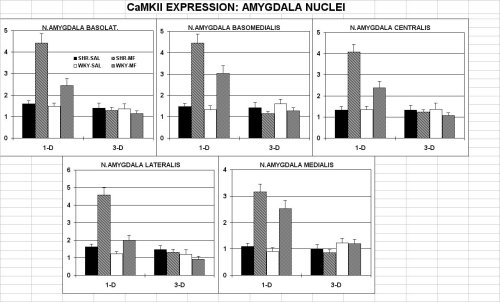 Fig. 2
Fig. 2
In addition MP treatment increased the expression of CaMKII in all nuclei of both lines. This effect was larger in the SHR than WKY, but faded out to base-line level three days following last drug injection in both rats lines
3. EXPRESSION OF c-FOS
Light microscope and computer-assisted high resolution image analysis revealed (i) a higher level of transcription factor c-FOS positive elements in both drug-treated groups (figure 3). His effect pertained to all areas and was more pronounce d in the SHR, but spared the nucleus accumbens pole and core, and the ventral pallidum in the WKY. (ii) In particular, in the SHR the drug reversed the reduced expression seen in the ventral pallidum and the cingulate cortex of vehicle-treated controls. ( iii) The beneficial effect of Methylphenidate faded out within three days from the last injection (data not shown).
Within the amygdala complex, as shown in figure 3, under basal conditions the SHR showed a higher number of c-FOS elements in basomedial and medial amygdaloid nuclei, and peririnal cortex( data not shown)
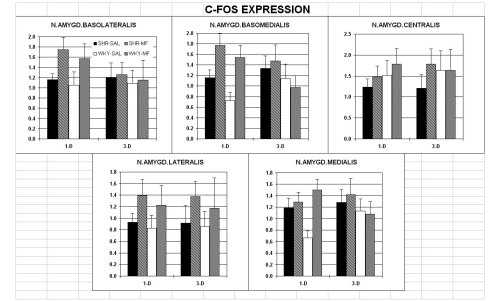 Fig. 3
Fig. 3
In addition, MP sharply increases the number of "activated" neurons in all amygdala nuclei with no effect in the central nucleus (output way) in both lines. In the medial nucleus of the amygdala, a dramatic effect is evident in WKY but not in SHR. In cingulate and perirhinal cortices, MP induces a significant increase in c-FOS positive neurons in booth strains, whereas in frontal cortex the increase is shown only in WKY (data not shown). Thus reaching the basal expressed in level of SHR which show a two-fold increase in the number of positive elements. These effect faded out 3 days following the last injection
4. REGIONAL CROSS-CORRELATIONS FOR C.O. ACTIVITY :
The degree of cross-talk was tested by correlating within each group individual levels of C.O. activity among different brain areas, as described earlier by Cada et al. 10, which is shown Fig. 4. In fact, averaging out the indi vidual correlation coefficients by Pearson, after "z-score" transformation, revealed that the naive SHR have an average correlation score higher than the WKY (WKY: 0.420 ± 0.102; SHR: 0.782 ± 0.041; t= 2.333; df= 54; 0.025 < 2p < 0.05 by t-test for non-paired data). Further, SHR are more "synchronized" among the different areas of the basal forebrain than WKY rats. In fact, the WKY differed from the SHR mainly in the cross-talk between the medial and lateral frontal cortices and the dorsal and ventr al portions of the CPU, and between the latter and the ventral pallidum. In contrast, under a general background of synchronization, the SHR showed a small degree of cross-talk only between the core and the shell of the accumbens and the ventral pallidum. Finally, there were significant differences pertaining to the gender and to the brain side. In fact, the average cross-talk indexed by the correlation score within the left and the right hemisphere was twice as much in SHR males compared to fem ales on both sides. In addition, the cross-talk between hemispheres, similar under basal conditions, increased following training in male SHR only. In contrast, the cross-talk involving only pairs of region on both hemispheres was similar across ra t line and treatment.
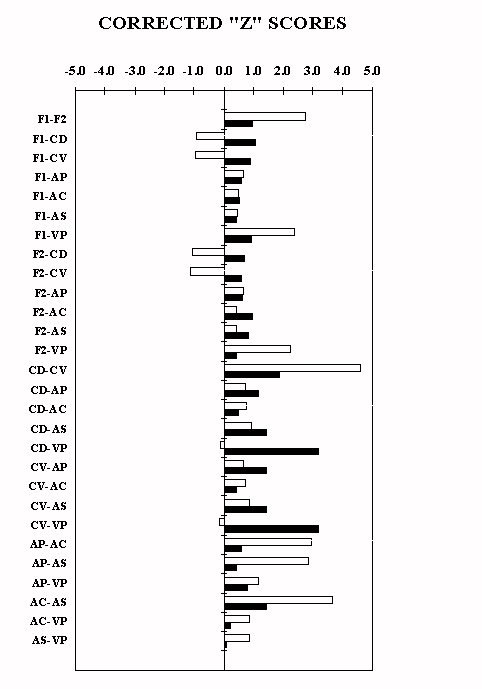 Fig. 4
Fig. 4
Discussion and Conclusion
Within the constraints of a reductionistic approach, the studies with CaMKII and c-FOS, as markers of neuronal activity, were aimed at verifying the modifiability of the segmental defect which demonstrated in the anterior forebrain of the SHR (Sadile A.G., this symposium). This was tested by subchronic treatment during 15 days with methylphenidate 6.
The data on CaMKII and c-FOS expression indicate that a subchronic treatment with methylphenidate, a drug which increases the efficiency of the mesolimbic dopamine system by increasing the availability of dopamine at receptor sites. In turn this leads to an increase the number of activated neurons and modules available for the integration of information between limbic and motor systems (Di Chiara G, this symposium) . This effect pertains not only the restricted segment of the anterior forebrain, wh ich has been recently hypotesized to be defective in the SHR, but also at neural sites distant from this restricted segment. In particular within the amygdala complex, there was a differential effect of drug treatment on CaMKII and c-FOS: In fact, while C aMKII demonstrated a significant increase in all amygdala nuclei, though larger in the SHR, c-FOS showed a significant drug effect which excluded the medial nucleus for SHR, the input processing unit.
This finding is of great interest because the medial nucleus of amygdala represents the entry to the amygdala complex. 11. The differential increase induced by MP treatment in CaMKII but not in c-FOS might be due to a more complex integrati on conveying information onto the neurons of the medial nucleus of the amygdala
However, the MP beneficial effect is short-lasting as it fades out within three days from drug withdrawal in both rats lines and for both CaMKII and c-FOS. Therefore, it appears that both markers of neuronal activity monitor remodeling of neural networ ks in this animal models of ADHD in a time-dependent, reversible manner.
Further, the results obtained in the amygdaloid complex suggest that the segmental defect yields network consequences in the so-called " extended amygdala" to whom the nucleus accumbens belongs 12. Thus, the data on the amygdaloid complex of animal model of ADHD might also explain the emotional problems that ADHD children show in a significant number of cases (see Sergeant J.A, this symposium).
Finally, another way to test remodeling of neural networks, using molecular probes such as DA receptors and C.O. activity, was to monitor post-hoc the cross-talk among different neural sites distant from the hypotesized segmental defect of the anterior forebrain. This was accomplished by regional cross correlation analysis, according to a procedure similar to that largely used in neurophysiology. This analysis demonstrates an altered cross-talk in the SHR forebrain under basal conditions, which was mo dified by MP treatment.
In perspective, the findings on altered cross-talk in the forebrain of the SHR appear very promising in that, if valitated in humans, they could pivot functional neuro-imaging techniques based on MRI or PET, for an early diagnosis and treatment of ADHD cases both in children as well as in adults.
SUMMARY
Molecular biology and light-microscope imaging techniques have been used to map in the anterior forebrain of an animal model of ADHD, the juvenile pre-hypertensive male SHR and WISTAR-KYOTO (WKY) controls, the spatial distribution of markers of neuron al activity such as (i) Dopamine (DA) D-1 and D-2 receptors by quantitative autoradiography, (ii) the Ca2+/Calmodulin-dependent protein kinase II (CaMKII), (iii) the transcription factor c-FOS, by ICC, and (iv) Cytochrome-oxydase (C.O.) .
Subcronic Methylphenidate (MP) treatment yielded a remodeling of neural networks in the cortico-striato-pallidal-system as well as in the amygdaloid complex. This remodeling was also monitored by using regional cross correlation analysis, which demons trated an altered cross-talk among different neural sites.These findings should promote functional neuroimaging techniques for an early diagnosis and treatment of ADHD cases.
References
This project has been supported partially by EU HCM contract and Telethon-Italy grant E.513.
1. Aspide, R., Gironi Carnevale, U. A., Sergeant, J. A. & Sadile, A. G. Behav. Brain Res. 95, 123-133 (1998).
2. Carey, M. P., Diewald, L., Papa, M., et al. Behav. Brain Res. 94, 173-185 (1998).
3. Papa, M., Sagvolden, T., Sergeant, J. A. & Sadile, A. G. Neuroreport. 7, 3017-3020 (1996).
4. Papa, M., Sergeant, J. A. & Sadile, A. G. Synapse (1996).
5. Papa, M., Sergeant, J. A. & Sadile, A. G. Behav. Brain Res. 94, 187-195 (1998).
6. Liu, Y., Peter, D., Merickel, A., Krantz, D., Finn, J. P. & Edwards, R. H. Behav. Brain Res. 73, 51-58 (1996).
7. List, S. J. & Seeman, P. Proc. Natl. Acad. Sci. U. S. A. 78, 2620-2624 (1981).
8. Gonzalez-Lima, F. & Jones, D. Brain Res. 660, 34-49 (1994).
9. Edwards, A. L. Statistical Methods fot the Behavioral Sciences ( Holt, Rinehart and Winston, New York, 1967).
10. Ling, M. R., Swinyer, L. J., Jarratt, M. T., et al. J. Am. Acad. Dermatol. 38, S95-102 (1998).
11. Pitkanen, A., Savander, V. & LeDoux, J. E. Trends Neurosci. 20, 517-523 (1997).
12. Zahm, D. S. & Brog, J. S. Neuroscience 50, 751-767 (1992).
13. Papa, M., Sagvolden, T., Sergeant, J. A. & Sadile, A. G. Behav. Brain Res. (1997).
| Discussion Board | Previous Page | Your Symposium |