Invited Symposium: What Can Genetic Models Tell Us About Attention-Deficit Hyperactivity Disorder (ADHD)?
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Spontaneously hypertensive rats (SHR) have been proposed as an animal model mimicking the behavioral defects of attention deficit/hyperactivity disorder (AD/HD) 1. Since this rat strain also exhibits severe hypertension, it is not possible to exclude that any behavioral deficits in this strain may in some way be attributable to altered cardiovascular function, as opposed to a true "psychological" deficit. Wistar-Kyoto-derived Hyperactive (WKHA) rats constitute an inbred strain that has been derived from the progeny of hybrid WKY/SHR F2 crosses 2. The activity score obtained with WKHA in a forced exploration test in a novel environment is as high as in SHR, but systolic blood pressure is normal as in Wistar-Kyoto (WKY). This strain has therefore been proposed by some as a possible alternative to SHR to study hyperactivity disorders.
To further verify the utility of the WKHA strain, we have performed a genetic characterization , comparing WKHA rats to their parental WKY and SHR strains 3. We also performed behavioral tests comparing WKHA to 2 other strains, in order to test the suitability of WKHA as a model for AD/HD. In addition to activity level in a forced exploration test in a novel environment, we examined these strains in the prepulse inhibition test of the acoustic startle reflex. This test assesses sensorimotor gating, or the extent to which a weak acoustic stimulus draws cognitive processing away from a more pronounced acoustic stimulus. This test is thought to be dependent upon processes involved in stimulus selection and stimulus control over behavior, processes that may be aberrant in AD/HD. Finally, given the effectiveness of the psychomotor stimulant methylphenidate in the treatment of AD/HD, we explored the effects of this compound on the performance of these three strains in each behavioral test.
Materials and Methods
Genetic characterization:
Genomic DNA was extracted from the spleens of WKHA/Edh, WKY/Nih and SHR/Nih rats. To determine the degree of genetic relatedness of the strains, DNA fingerprinting was performed by Southern blot analysis of Hinf I- or Alu I-digested DNA. By using 6 different probes corresponding to 2 minisatellite sequences and 4 microsatellite sequences, we visualized an average total of 135 bands for each strain. The same DNA samples were analyzed for the detection of simple sequence length polymorphisms (SSLP) by PCR amplification with a total of 216 primer pairs.
Behavioral tests:
Three different strains were used for behavioral tests: WKHA, WKY and outbred Wistar rats (WIST). All rats were males used at 12 weeks of age. Forced exploration tests in a novel environment were performed in a square Lucite cage (30 x 30 cm) equipped with 4 sets of light beams. The animals were placed in the cage for a total of 10 min, and locomotor activity was measured by calculating the number of times the light beams were interrupted.
Acoustic startle responses and prepulse inhibition were measured in two startle chambers consisting each of Plexiglas walls mounted on a Plexiglas base within a sound-attenuating chamber. A piezoelectric strain meter attached to the base transduced the startle response. Stabilimeter readings were rectified, digitized on a 4095 scale, and recorded by a computer. A speaker located in the ceiling of the sound attenuating chamber presented all acoustic stimuli and maintained a constant background noise level of 70 dB. Startle reactivity was assessed by exposing animals to a 30 msec, 120 dB acoustic stimulus alone. An average of fifty 1-msec readings, beginning at the onset of the startle stimulus, was used as the dependent variable.
Prepulse inhibition (PPI) of acoustic startle responses was measured by having the 120 dB startle stimulus preceeded by a 30 msec prepulse stimulus, which terminated 70 msec before the onset of the startle stimulus. The intensity of the prepulse stimulus varied from between 3 and 15 dB above the background noise level in 3 dB. A test session consisted of placing the animals in the startle chamber for a 5-min acclimatization period after which they were exposed to a total of 37 trials separated by variable inter-stimulus-intervals that averaged 15 sec. The first 2 initial trials were startle trials. Results of the very first 2 trials were discarded as animals generally over-react to them. Over the last 35 trials, animals were exposed to an additional 10 startle trials, and to 5 trials at each of the 5 prepulse intensities. These trials were presented randomly, with the one restriction that no more than two trials of the same type could occur in succession. For data analysis, the average of the last 10 startle trials was taken as the measure of startle reactivity for each animal. We also averaged the 5 trials taken at each of the 5 prepulse intensities, and then expressed these values as a percentage of the average reactivity for the 10 startle trials, using the formula: [(startle-prepulse)/startle] x 100.
In pharmacological experiments, the animals received either methylphenidate (5 mg/kg b.w.) or saline s.c. 30 min before the behavioral test.
Results
The table below represents the % value of genetic relatedness of various strains, as determined by DNA fingerprinting.
|
SHR/Nih |
WKHA |
|
|
WKHA |
74% |
~ |
|
WKY/Nih |
62% |
85% |
It appears that WKHA were more closely related to either SHR of WKY than the degree to which the 2 parental strains were related to each other. By SSLP analysis, we found that 108 out of the 216 markers tested revealed polymorphisms between SHR and WKY. These 108 markers covered the whole genome with an average spacing of 12 cM. In WKHA, 36 % of the polymorphic markers were found to originate from SHR.
For virtually all alleles detected with the 216 markers in WKHA, a similarly-sized allele could be detected in the parental WKY or SHR strains. Furthermore, we detected virtually no heterozygosities of SSLP marker alleles in WKHA, confirming that all alleles are identical.
Fig. 1 (below) shows how the three strains compared in terms of activity in a forced exploration test and the amplitude of their startle response to an acoustic stimulus. In both cases, the amplitudes of the responses were lower in WKY than in WKHA. However, the responses of WKHA were lower than that of Wistar rats, and of similar magnitude than that of Lewis rats (data not shown).
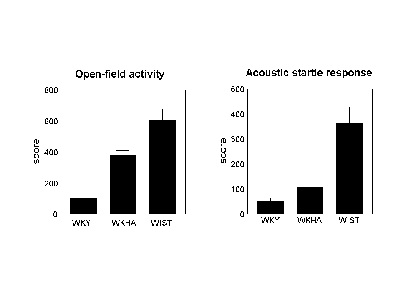
Fig.1: Comparisons of locomotor activity and startle responses in 3 rat strains.
Fig. 2 (below) shows how the three strains compared in terms of their inhibition responses to prepulses of 5 different amplitudes. The amplitudes of the inhibition responses were comparable in WKHA and Wistar. In contrast, PPI was lower in WKY than in both other strains for each of the 5 different prepulse intensities.
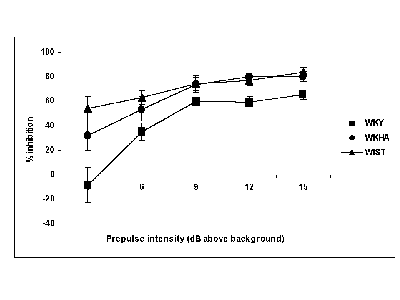
Fig. 2: PPI in 3 rat strains at 5 different prepulse intensities.
Finally, we tested whether methylphenidate (a drug used in humans to treat AD/HD) might have effect on the behavior of WKHA that might indicate that this strain could be used as a model of AD/HD. Fig. 3 (below) shows that methylphenidate increased the locomotor activity of all 3 strains in the forced exploration test. The amplitude of the effect was similar in WKHA and Wistar rats, but both strains responded to a much lower extent than what was observed in WKY. The PPI response of both in WKHA and Wistar rats was reduced (and to a similar extent) by methylphenidate. However, the drug had a much different effect in WKY, where it increased the PPI response at different prepulse intensities (Fig. 3 shows the results for 2 different intensities).
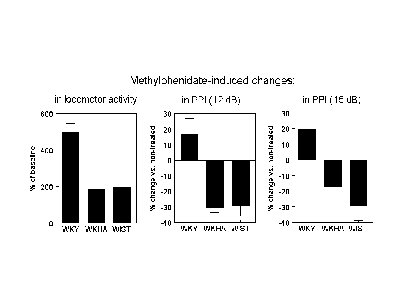
Fig. 3: Methylphenidate-induced changes in locomotor activity and in PPI responses.
Discussion and Conclusion
The genetic tests showing that all alleles were identical in WKHA confirmed that this strain is truly inbred. Comparison of the sizes of the alleles with the parental strains also confirmed that the genetic background of WKHA constitutes a true mix of the backgrounds of the parental WKY and SHR. As such, these data reveal the usefulness of this particular strain for genetic investigations. However, does it constitute a model of AD/HD? When open-field forced exploration tests are performed, WKHA are indeed more active than WKY. However, this is not sufficient to label the WKHA as "hyperactive", since WKHA display activity levels that are comparable (or even inferior) to that of many other strains. This is similar to previous reports, where WKY was found to be less active than SHR, but the latter was found to be either as or less active than many other strains 4, 5. Thus, the difference in activity between WKHA and WKY might be due more to the hypo-activity of WKY than to the hyperactivity of WKHA. It also follows that in the previous study linking a genetic locus to activity in WKHA/WKY crosses 6, the incriminated gene is more likely to be a hypo-activity gene from WKY than a hyperactivity gene from WKHA.
The PPI test is strongly linked to central dopaminergic function 7, and constitutes a test of sensory-motor gating that evaluates how the animal selects and is controlled by environmental cues. Both characteristics make it an interesting test to evaluate whether rat strains exhibit behavioral responses that might mimic AD/HD. PPI was stronger in WKHA than in WKY, and of similar amplitude as in Wistar rats. There was, therefore, no clear indication of a deficit in WKHA rats. More importantly, if the WKHA strain is to be considered a valid model of AD/HD, any behavioral deficits observed in this strain should be sensitive to methylphenidate administration. However, we did not find that methylphenidate affected locomotor behavior or PPI in a manner consistent with favorable effects on AD/HD symptoms.
Intriguingly, if any deficit was present, it could be seen in WKY. Indeed, this strain exhibited lower levels of PPI, and it was improved by methylphenidate administration. Arguably, the true significance of this result must be interpreted cautiously. Indeed, prepulse inhibition is thought to be an index of a simpler pre-attentional process, as opposed to the more intricate and complex process that constitutes "attention". This concession notwithstanding, it is clear that one function common to both pre-attentional and attentional processes is the selection and filtering of information, which is clearly deficient in AD/HD. In humans, there is only one study to date that has tested sensory-motor gating in children suffering from AD/HD 8. Whereas this study did report a deficit in prepulse inhibition, this deficit was observed only in subjects co-morbid for AD/HD and a movement (tic) disorder. Consequently, the issue of whether AD/HD alone gives rise to deficient prepulse inhibition requires additional experimental work.
Altogether, we conclude that the behavioral differences between WKY and WKHA can be more readily explained by deficits present in WKY than by unusual characteristics of WKHA. The WKHA, despite its interesting genetic characteristics, does not appear to represent a valid model of AD/HD.
References
- Sagvolden T, Metzger MA, Schiorbeck HK, Rugland A-L, Spinnangr I, Sagvolden G: The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and psychomotor stimulants. Behav.Neur.Biol. 1992;58:112-1992
- Hendley ED, Ohlsson WG: Two new inbred rat strains derived from SHR: WKHA, hyperactive, and WKHT, hypertensive, rats. Am.J.Physiol. 1991;261:H583-H589
- Deschepper CF, Prescott G, Hendley ED, Reudelhuber TL: Genetic characterization of novel strains of rats derived from crosses between Wistar-Kyoto and spontaneously hypertensive rats and comparisons with their parental strains. Lab.Anim.Sci. 1997;47:638-646
- McCarthy R: Stress, behavior and experimental hypertension. Neurosci.Biobehav.Rev. 1983;7:493-452
- Tilson HA, Chamberlain JH, Gylys JA, Buyinski JP: Behavioral suppressant effect of clonidine in strains of normotensive and hypertensive rats. Eur.J.Pharmacol. 1977;43:99-105
- Moisan M-P, Courvoisier H, Bihoreau M-T, Gaugier D, Hendley ED, Lathrop M, James MR, Mormède P: A major quantitative trait influences hyperactivity in the WKHA rat. Nature Genetics 1996;14:471-472
- Swerdlow NR, Braff DL, Taaid N, Geyer MA: Assessing the validity of an animal model of deficient sensorimotor gating in schizoprhrenic patients. Arch.Gen.Psychiatry 1994;51:139-154
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M: Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol.Psychiatry 1996;39:33-41
| Discussion Board | Previous Page | Your Symposium |