Invited Symposium: Hypertension III: Flow-Induced Vascular Remodeling
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
INTRODUCTION AND MODEL
Luminal enlargement of collaterals has been well documented(Coyle, 1984; Conrad et al., 1971; Schaper et al., 1988; Paskins-Hurlburt & Hollenberg, 1992; White et al., 1992; Lehman et al., 1991). However, the primary stimulus responsible for collateral evolvement remains controversial. Furthermore, the specific mechanisms mediating the wall remodeling associated with progressive luminal expansion are largely unknown. Although growth factors produced by ischemic tissues(Hashimoto et al., 1994; Schaper, 1993a) or monocytes(Schaper, 1993b) may participate in collateral development, there is substantial evidence to suggest that shear stress is a primary stimulus for arterial growth(Langille & O'Donnell, 1986; Kamiya & Togawa, 1980; Zarins et al., 1987) and collateral enlargement(Unthank et al., 1996b; Unthank et al., 1996a; Ito et al., 1997). This structural regulation of vascular diameter by blood flow has been shown to require an intact endothelium(Langille & O'Donnell, 1986; Tohda et al., 1992) and cell culture studies with molecular biology techniques have also demonstrated that shear stress can induce the transcription of endothelial genes for many growth factors (see references (Resnick & Gimbrone, Jr., 1995; Skalak & Price, 1996) for review).
In this review, a summary of studies performed in our laboratory investigating remodeling of small resistance arteries after abrupt elevation of blood flow will be presented. The model utilized in these studies has been previously described(Unthank et al., 1996b; Unthank et al., 1996a). It permits paired observations of arterial diameters along a well-defined collateral pathway, before and at various times after flow elevation due to arterial ligation. The techniques used with this model for repeated observation do not alter microvascular growth or remodeling (Unthank & Bohlen, 1987; Unthank & Bohlen, 1988; Unthank et al., 1996b). Arterial blood flow at the beginning of the collateral pathway is increased ~275% (Figure 2) following the creation of the model. Wall shear stress is initially increased ~175% (Figure 3). As luminal expansion occurs, blood flow in the collateral artery remains elevated but wall shear rates are restored to normal levels. Because normal tissue perfusion is maintained and arterial pressure at the site of primary adaptation is not altered(Unthank et al., 1996b), shear stress is considered to be the major stimulus for the initial arterial adaptation in this model. Additional details regarding this model are given in Figures 1-3. The focus of this review will be upon changes in the intima and media during the progression of collateral development. Wall remodeling will be specifically examined at time points when wall shear remains elevated (1 wk), has been restored to normal levels (4 wk), and a significantly later time (12 wk) when the adaptations to elevated shear are assumed to be at or near completion.
 Click to enlarge
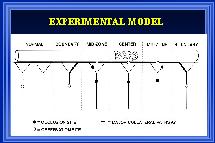
Fig. 1: A collateral arterial pathway was created as previously described (Unthank et al., 1996b) by ligating 3-4 sequential mesenteric arteries supplying blood flow to the terminal ileum of ~200g Wistar rats. Solid black circles in the diagram illustrate the sites of ligation. Between the two boundary collaterals, there were ~50 large arterioles over a distance of ~10 cm. The open circles indicate sites of diameter measurement at defined regions (boundary, mid-zone, center) along the collateral dependent pathway as well as at control or normal areas.
Click to enlarge
Fig. 1: A collateral arterial pathway was created as previously described (Unthank et al., 1996b) by ligating 3-4 sequential mesenteric arteries supplying blood flow to the terminal ileum of ~200g Wistar rats. Solid black circles in the diagram illustrate the sites of ligation. Between the two boundary collaterals, there were ~50 large arterioles over a distance of ~10 cm. The open circles indicate sites of diameter measurement at defined regions (boundary, mid-zone, center) along the collateral dependent pathway as well as at control or normal areas.
 Click to enlarge
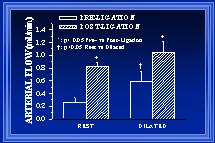
Fig. 2: In acutely studied rats, blood flow in the ileal arteries at the boundary region was measured before and after arterial ligation under resting (control) and maximally dilated conditions.
Click to enlarge
Fig. 2: In acutely studied rats, blood flow in the ileal arteries at the boundary region was measured before and after arterial ligation under resting (control) and maximally dilated conditions.
 Click to enlarge
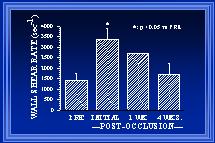
Fig. 3 After arterial ligation, wall shear rate (WSR) is initially elevated ~175%(Unthank et al., 1996b). After one week, the collateral diameter is increased ~30% and WSR is estimated to remain elevated ~80%(Unthank et al., 1996a). By 4 weeks, additional luminal enlargement has occurred and WSR is similar to both pre-ligation WSR and WSR in normal arteries of the same animals(Unthank et al., 1996a). At 12 weeks (data not shown), the WSR in collateral arteries remains similar to WSR in normal arteries (Fath et al., 1998).
Click to enlarge
Fig. 3 After arterial ligation, wall shear rate (WSR) is initially elevated ~175%(Unthank et al., 1996b). After one week, the collateral diameter is increased ~30% and WSR is estimated to remain elevated ~80%(Unthank et al., 1996a). By 4 weeks, additional luminal enlargement has occurred and WSR is similar to both pre-ligation WSR and WSR in normal arteries of the same animals(Unthank et al., 1996a). At 12 weeks (data not shown), the WSR in collateral arteries remains similar to WSR in normal arteries (Fath et al., 1998).
COLLATERAL LUMINAL EXPANSION AND SHEAR
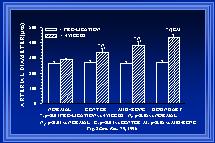
In our initial studies, inner arterial diameters were measured at the same locations along the collateral pathway before and at 1 or 4 weeks post-ligation. The measurements were made with the arteries maximally dilated so that changes in diameter would indicate alterations in structure (i.e. luminal expansion or retraction) rather than tone. These measurements were grouped according to their location along the collateral path (see Figure 1). At both 1wk (Unthank et al., 1996b) and 4wk(Unthank et al., 1996a), the luminal expansion was greatest at the beginning of the collateral pathway. At four weeks, the increases in inner arterial diameter along the collateral path (Figure 4) were 64+/-6% at the beginning (boundary), 47+/-9% at the middle (mid-zone), and 25+/-9% at the end (center). Because the pre-ligation diameters of the arteries were similar at the various locations (Figure 2) and blood flow within the arteries would decrease from the boundary to center regions as the arterioles of the intestine received their input from the marginal artery, wall shear would be expected to decrease from the boundary to the center. Thus, these data demonstrate that the largest diameter increases occur at the sites where wall shear forces are the most increased.
 Click to enlarge
Fig. 4 Means of animal averages of actual inner arterial diameters before and four weeks after arterial ligation are shown for the four regions studied (normal or control areas and along the collateral pat at the boundary or beginning, middle or mid-zone, and the end or center). Note that the pre-ligation diameters were all similar and that no increase was observed in the arteries at the normal tissue regions.
Click to enlarge
Fig. 4 Means of animal averages of actual inner arterial diameters before and four weeks after arterial ligation are shown for the four regions studied (normal or control areas and along the collateral pat at the boundary or beginning, middle or mid-zone, and the end or center). Note that the pre-ligation diameters were all similar and that no increase was observed in the arteries at the normal tissue regions.
WALL REMODELING
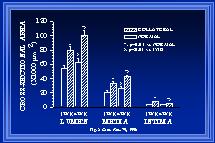
During changes in luminal diameter, components of the intima, media and adventitia must be reorganized to allow the structural diameter alterations. Such reorganization may occur with increases, decreases, or no net change in the amount of cellular and noncellular materials within each wall layer. Although wall thinning occurs in coronary collaterals after gradual arterial occlusion(White et al., 1992), our observations indicate that intimal and medial cross-sectional thickness and areas are increased in the collateral at the boundary location. Figure 5 demonstrates the increase in wall areas. Our observations are consistent with those in collateral cerebral arteries after abrupt arterial occlusion(Lehman et al., 1991). In a model similar to ours, Pourageaud and De Mey(Pourageaud & De Mey, 1997) have observed adventitial cross-sectional area of mesenteric arteries to decrease with chronic reductions in blood flow but not to be elevated when blood flow and shear are elevated. It is important to note that at all times studied, the relationship between medial thickness and luminal radius in collaterals has not been altered from that of control arteries as shown in Figure 6(Unthank et al., 1996a; Fath et al., 1998). Both our work and that by Pourageaud and De Mey(Pourageaud & De Mey, 1997) demonstrate that, in small resistance arteries subjected to abrupt elevation of blood flow, circumferential wall stress is maintained constant as luminal expansion occurs to normalize wall shear stress.
 Click to enlarge
Fig. 5 The means of the animal averages of luminal, medial, and intimal cross-sectional areas are shown for normal and collateral arteries (boundary region) one and four weeks after creation of the model.
Click to enlarge
Fig. 5 The means of the animal averages of luminal, medial, and intimal cross-sectional areas are shown for normal and collateral arteries (boundary region) one and four weeks after creation of the model.
 Click to enlarge
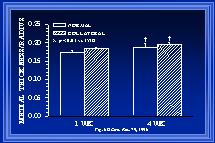
Fig. 6 The means of the animal averages of medial thickness to luminal radius relationship, calculated from the cross-sectional areas of Figure 5, are shown. Additional data from 12-week animals indicates that the ratio of medial thickness to luminal radius is similar between control and collateral arteries at all times studied.
Click to enlarge
Fig. 6 The means of the animal averages of medial thickness to luminal radius relationship, calculated from the cross-sectional areas of Figure 5, are shown. Additional data from 12-week animals indicates that the ratio of medial thickness to luminal radius is similar between control and collateral arteries at all times studied.
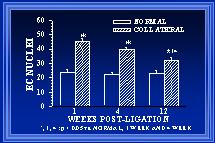
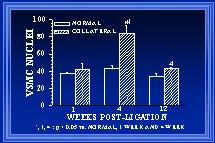
Within each area, both cellular and extracellular components contribute to the total cross-sectional wall area. Nuclear numbers in the intima and media of arterial cross-sections were counted to determine if either cellular proliferation or regression was associated with the increases in wall area during collateral luminal expansion associated with elevated wall shear. Figure 7 demonstrates that there was a significant increase (>90%) in endothelial cell number within the first week after flow elevation. Calculations of endothelial cell density (number nuclei per luminal circumference) indicated that intimal cell density was increased >60% at one week(Unthank et al., 1996a). By week 12, the number of cross-sectional endothelial cell nuclei had significantly decreased and intimal cell density was similar between control and collateral arteries. In the media, an increase in medial cell number was not observed until week 4 (Figure 8). Consequently, medial cell density was decreased ~30% after 1 week. The 80% increase in medial cell nuclei at four weeks restored medial cell density to control levels for this time point. However, by week 12, cross-sectional medial cell number in collaterals had decreased and medial cell density was slightly (~15%) decreased at this time. In a similar model in which wall shear is initially increased ~100% rather than 175%, Tulis et al (Tulis et al., 1998) have shown that medial cell density is increased within 1 week. We believe that this indicates that the magnitude of the wall shear elevation influences the remodeling within each wall layer during luminal expansion.
 Click to enlarge
Fig. 7 The number of endothelial cells (EC), determined from the number of nuclei counted in arterial cross-sections, is significantly elevated in collateral arteries. At one week, there are 90% more EC in the collateral than normal arteries. At 12 weeks, there are significantly fewer EC nuclei in the collaterals than at 1 or 4 weeks (32±2 vs 45±2 and 40±2).
Click to enlarge
Fig. 7 The number of endothelial cells (EC), determined from the number of nuclei counted in arterial cross-sections, is significantly elevated in collateral arteries. At one week, there are 90% more EC in the collateral than normal arteries. At 12 weeks, there are significantly fewer EC nuclei in the collaterals than at 1 or 4 weeks (32±2 vs 45±2 and 40±2).
 Click to enlarge
Fig. 8 The number of smooth muscle cells (SMC), determined from the number of nuclei counted in arterial cross-sections, is shown. Although at 4 weeks, the number of SMC in collaterals is increased >80% relative to normal arteries, the number of SMC in 12 week collaterals is decreased to be similar to normal arteries.
Click to enlarge
Fig. 8 The number of smooth muscle cells (SMC), determined from the number of nuclei counted in arterial cross-sections, is shown. Although at 4 weeks, the number of SMC in collaterals is increased >80% relative to normal arteries, the number of SMC in 12 week collaterals is decreased to be similar to normal arteries.
CONCLUSIONS AND DISCUSSION
1. Wall shear is a major stimulus for vascular growth and remodeling. This has been demonstrated throughout the vasculature from large conduit arteries (Kamiya & Togawa, 1980; Zarins et al., 1987; Langille, 1995) to arterioles (Wang & Prewitt, 1993; Wang & Prewitt, 1991). We also believe that shear forces are important in collateral artery development, especially that which results from an abrupt occlusion. Under appropriate conditions, ischemia(DeFily & Chilian, 1993) and monocytes(Ito et al., 1997) may also have important roles in collateral development. The observation that the medial thickness to luminal radius relationship is preserved during luminal expansion after abrupt arterial occlusion suggest that forces related to circumferential wall stress are also of major importance during the remodeling processes in these vessels.
2. The magnitude and rate of luminal expansion is correlated with the degree of elevation of wall shear forces. This is supported by our measurements of diameter increases along the collateral pathway (Figure 4).
3. The nature of the remodeling process varies with shear forces. Not only are different cellular processes involved depending upon whether shear is chronically increased or decreased(Langille, 1995), but net changes in endothelial and smooth muscle cell number appear to be dependent upon the magnitude of the shear increase. With an initial increase in shear of ~175%, only endothelial cell number is increased at one week (Figures 7 and 8). Work by Tulis et al indicates that both endothelial and smooth muscle nuclear numbers are increased at one week when shear is elevated by ~100%(Tulis et al., 1998). We hypothesize that the abrupt elevation of blood flow increases the expression of shear-regulated growth factors within the endothelium. These growth modulators act in an autocrine and paracrine manner to influence remodeling events. As luminal expansion occurs and wall shear forces decrease to normal levels, the balance between growth modulators within the arterial wall is continuously adjusted. This balance between various growth promoters and inhibitors for the cells within the wall layers determines the nature of the remodeling at a specific time.
4. Our work also demonstrates that remodeling continues after wall shear forces have been normalized. It appears that the initial response to an abrupt elevation of wall shear produces an overabundance of cells within both the intima and media and that cellular regression/rarefaction occur during and after the restoration of wall shear force to normal.
Arterial remodeling can have profound influences on reactivity as demonstrated by Pourageaud and De Mey(Pourageaud & De Mey, 1997). In many ways, the remodeling that occurs in small resistance arteries appears to restore many wall parameters to normal values(Fath et al., 1998). As wall shear is returned to control levels, at 12 weeks the medial thickness to luminal radius relationship is preserved, endothelial cell density is restored to normal, and medial cell density is close to normal. It is important to note, however, that these studies were conducted in relative young animals (8-10 weeks) without any known pathologies. As will be shown by data presented at this symposium by Tuttle, both aging and vascular diseases may have substantial impact upon the remodeling observed with abrupt elevation of wall shear forces.
REFERENCES
- CONRAD, M.C., ANDERSON, J.L. & GARRETT, J.B. (1971). Chronic collateral growth after femoral artery occlusion in the dog. Journal of Applied Physiology 31, 550-555.
- COYLE, P. (1984). Diameter and length changes in cerebral collaterals after middle cerebral artery occlusion in the young rat. Anatomical Record 210, 357-364.
- DEFILY, D.V. & CHILIAN, W.M. (1993). Methods for assessing coronary collateral growth: Insights into mechanisms responsible for collateral development. In Collateral Circulation: Heart, Brain, Kidney, Limbs, eds. SCHAPER, W. & SCHAPER, J., pp. 29-40. Boston: Kluwer Academic Publishers.
- FATH, S.W., BURKHART, H.M., MILLER, S.C., DALSING, M.C. & UNTHANK, J.L. (1998). Wall remodeling after wall shear rate normalization in rat mesenteric arterial collaterals. Journal of Vascular Research 35, 257-264.
- HASHIMOTO, E., OGITA, T., NAKAOKA, T., MATSUOKA, R., TAKAO, A. & KIRA, Y. (1994). Rapid induction of vascular endothelial growth factor expression by transient ischemia in rat heart. American Journal of Physiology 267, H1948-H1954.
- ITO, W.D., ARRAS, M., WINKLER, B., SCHOLZ, D., SCHAPER, J. & SCHAPER, W. (1997). Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circulation Research 80, 829-837.
- KAMIYA, A. & TOGAWA, T. (1980). Adaptive regulation of wall shear stress to flow change in the canine carotid artery. American Journal of Physiology 239, H14-H21.
- LANGILLE, B.L. (1995). Blood flow-induced remodeling of the artery wall. In Flow-Dependent Regulation of Vascular Function, eds. BEVAN, J.A., KALEY, G. & RUBANYI, G.M., pp. 277-299. New York: Oxford University Press.
- LANGILLE, B.L. & O'DONNELL, F. (1986). Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science (Washington) 231, 405-407.
- LEHMAN, R.M., OWENS, G.K., KASSELL, N.F. & HONGO, K. (1991). Mechanism of enlargement of major cerebral collateral arteries in rabbits. Stroke(Dallas) 22, 499-504.
- PASKINS-HURLBURT, A.J. & HOLLENBERG, N.K. (1992). "Tissue need" and limb collateral arterial growth: skeletal contractile power and perfusion during collateral development in the rat. Circulation Research 70, 546-553.
- POURAGEAUD, F. & DE MEY, J.G.R. (1997). Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. American Journal of Physiology 273, H1669-H1706
- RESNICK, N. & GIMBRONE, M.A., Jr. (1995). Hemodynamic forces are complex regulators of endothelial gene expression. The FASEB Journal 9, 874-882.
- SCHAPER, W. (1993a). Angiogenesis in the adult heart. Basic Research in Cardiology 86(Suppl 2), 51-56.
- SCHAPER, W. (1993b). Coronary collateral development: concepts and hypotheses. In Collateral Circulation: Heart, Brain, Kidney, Limbs, eds. SCHAPER, W. & SCHAPER, J., pp. 41-64. Boston: Kluwer Academic Publishers.
- SCHAPER, W., GORGE, G., WINKLE, B. & SCHAPER, J. (1988). The collateral circulation of the heart. Progress in Cardiovascular Diseases 31, 57-77.
- SKALAK, T.C. & PRICE, R.J. (1996). The role of mechanical stresses in microvascular remodeling. Microcirculation 3, 143-165.
- TOHDA, K., MASUDA, H., KAWAMURA, K. & SHOZAWA, T. (1992). Difference in dilatation between endothelium-preserved and - desquamated segments in the flow-loaded rat common carotid artery. Arteriosclerosis and Thrombosis 12, 519-528.
- TULIS, D.A., UNTHANK, J.L. & PREWITT, R.L. (1998). Flow-induced arterial remodeling in rat mesenteric vasculature. American Journal of Physiology 274, H874-H882.
- UNTHANK, J.L. & BOHLEN, H.G. (1987). Quantification of intestinal microvascular growth during maturation:techniques and observations. Circulation Research 61, 616-624.
- UNTHANK, J.L. & BOHLEN, H.G. (1988). Intestinal microvascular growth during maturation in diabetic juvenile rats. Circulation Research 63, 429-436.
- UNTHANK, J.L., FATH, S.W., BURKHART, H.M., MILLER, S. & DALSING, M.C. (1996a). Wall remodeling during luminal expansion of mesenteric arterial collaterals in the rat. Circulation Research 79, 1015-1023.
- UNTHANK, J.L., NIXON, J.C., BURKHART, H.M., FATH, S.W. & DALSING, M.C. (1996b). Early collateral and microvascular adaptations to intestinal artery occlusion in the rat. American Journal of Physiology 271, H914-H923.
- WANG, D.H. & PREWITT, R.L. (1991). Microvascular development during normal growth and reduced blood flow: introduction of a new model. American Journal of Physiology 260, H1966-H1972.
- WANG, D.H. & PREWITT, R.L. (1993). Alterations of mature arterioles associated with chronically reduced blood flow. American Journal of Physiology 264, H40-H44.
- WHITE, F.C., CARROLL, S.M., MAGNET, A. & BLOOR, C.M. (1992). Coronary collateral development in swine after coronary artery occlusion. Circulation Research 71, 1490-1500.
- ZARINS, C.K., ZATINA, M.A., GIDDENS, D.P., KU, D.N. & GLAGOV, S. (1987). Shear stress regulation of artery lumen diameter in experimental atherogenesis. Journal of Vascular Surgery 5, 413-420.
| Discussion Board | Previous Page | Your Symposium |