Pharmacology & Toxicology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Cyclic nucleotide phosphodiesterases (PDEs) catalyze the hydrolysis of the 3', 5'-cyclic nucleotides, cAMP and cGMP, to the corresponding nucleoside 5Õ-monophosphates. These cyclic nucleotides are intracellular second messengers which play important roles in a variety of signal transduction processes (1). Mammalian PDEs have been grouped into seven families based on their substrate affinities, inhibitor sensitivities, mode of regulation and amino acid sequence homologies (2). These families are PDE1: Ca2+/calmodulin-dependent PDE, PDE2: cGMP-stimulated PDE, PDE3: cGMP-inhibited PDE, PDE4: cAMP-specific PDE, PDE5: cGMP-specific PDE, PDE6: photoreceptor PDE, and PDE7: high affinity cAMP-specific, rolipram-insensitive PDE. Moreover, two additional families, PDE8 and PDE9, have been recently reported. PDE8 is a high affinity cAMP-specific, IBMX-insensitive PDE (3), and PDE9 is a high affinity cGMP-specific PDE (4, 5). Among all these PDE families, a conserved region of approximately 270 amino acids in the carboxyl-terminus apparently serves as the catalytic domain, whereas regions amino-terminal to the catalytic domain are divergent and presumably account for the distinctive regulatory properties unique to the individual families (6, 7).
The expressed sequence tags (ESTs) consist of partial "single pass" cDNA sequences from various tissues (8, 9). Analysis of the EST database is becoming a powerful approach to look for new members of gene families. In this study, we describe the cloning and characterization of novel human PDE isozymes through EST database search.
Materials and Methods
EST database search
The dbEST was searched with the nucleotide sequences or amino acid sequences of various PDE catalytic domains as queries using the data base search and analysis service on the World Wide Web (http://www.blast.genome.ad.jp/). The program used was Basic Local Alignment Search Tool (10).
Isolation and sequence of human PDE8A and human PDE8B cDNAs
The cDNA clones encoding the partial sequences of human PDE8A and human PDE8B were purchased from I.M.A.G.E. consortium and entirely sequenced. Next, 5' and 3' RACE-PCR (11) were carried out using these sequences on human brain cDNA commercially available for RACE-PCR (Clontech, Palo Alto, CA). For human PDE8A, the cDNA was amplified by PCR with one of the gene-specific primers based on an EST sequence (GenBank accession number N79954)(5'RACE-primer, 5'-ACAAA GGCAT CCCAA GCATC AAAAC AT-3'; 3'RACE-primer, 5'-CCTGC AGCAT CCCCA AATCC CAAAT CT-3') and an AP1 primer (CLONTECH), which is complementary to part of the cDNA adapter ligated at both ends of the cDNA. For human PDE8B, the cDNA was amplified by PCR with one of the gene-specific primers based on an EST sequence (GenBank accession number AA190710)(5'RACE-primer, 5'-GGCAG CATGG GTGGA GTTGT GGTAG G-3'; 3'RACE-primer, 5'-TTGGA AAGGA AAGAG TAAAG GGAAG C-3') and an AP1 primer. PCR reactions were performed in 50 ul reaction mixtures containing 0.2 mM of each dNTP, 10 pmol of each primer, 2.5 units of TAKARA LA Taq (Takara, Kyoto, Japan), and 1x buffer supplied with the polymerase. The PCR conditions were 5 cycles at 94C for 30 sec and 72C for 4 min, 5 cycles at 94C for 30 sec and 70C for 4 min, and then 25 cycles at 94C for 30 sec and 68C for 4 min. The amplification products were cloned into pGEM-T vectors (Promega, Madison, WI) by T-A ligation and sequenced on both strands using gene-specific and commercial primers.
Northern blot analysis
Multiple human tissue Northern blots and human RNA Master Blot of poly(A)+ RNA were purchased from Clontech Laboratories. Filters were hybridized with 32P-labeled PDE8A or PDE8B cDNA probe at 68C for 1 h in ExpressHyb hybridization solution (Clontech). After washing at 55C for 30 min in 0.2 x SSC and 0.1% SDS, filters were exposed to x-ray films at -80C with an intensifying screen.
Expression of fusion Proteins
The carboxyl-terminal 584 amino acids of PDE8B (bases 226-1977; amino acids 76-659) was amplified by PCR with specific primer set: 5'-GACGA CGACA AGATG GGCTA TCACA AAGGC GAG-3' and 5'-GAGGA GAAGC CCGGT TTAGC TGTCA GATGG AAGCC T-3' corresponding to bases 226-246 and 1960-1981 respectively. Sequences underlined are ligation independent cloning (LIC) sites for 5' and 3' end, respectively. PCR products were treated with T4 DNA polymerase, inserted into the corresponding LIC cloning sites of the bacterial expression vector pET32 (Novagen, Milwaukee, WI), and transformed into E. coli BL21(DE3) cells in accordance with the manufacturer's instructions. E. coli transformed with this plasmid were grown to an attenuation of 0.5-0.6 and then incubated with 0.5 mM isopropyl thiogalactopyranoside for 3 h at 37C. Cells were harvested by centrifugation and lysed for 15 min in a buffer containing 20 mM Tris (pH 8.0), 1 mM EDTA, and 2.2 mg/ml lysozyme. The cell lysate was then supplemented with 0.2 mM EGTA, 10 mM NaF, 50 mM benzamidine, 0.5 ug/ml leupeptin, and 0.7 ug/ml pepstatin.
Measurement of PDE activity
Extracts of E.coli cells bearing the PDE8B expression vector and control extracts of cells bearing the empty expression vector pET32 were assayed. Enzyme assays were performed as previously described (13) with some modifications. Samples were incubated with 1 uM [3H]cAMP or 1 uM [3H]cGMP for 10 min at 30C in a total volume of 0.5 ml containing 50 mM Tris (pH 8.0), 2 mM EGTA, 5 mM MgCl2, and 0.1 mg/ml bovine serum albumin. Reactions were terminated by boiling for 5 min. Fifty microliters of 2.5 mg/ml snake venom was added, followed by a 10 min further incubation at 30C. [3H]AMP or [3H]GMP were isolated on BioRad AG50W-X4 resin columns (Bio-Rad, Mississauger, ON, Canada) and 3H level in 10 ml of ACS scintillation fluid were analyzed using a Beckman LS 5000TA counter. Blank values were obtained using buffer alone. For inhibition studies, samples were assayed in the presence of 10-7 to 10-4 M of various inhibitors dissolved in dimethyl sulfoxide (DMSO), and the final solvent concentrations in the PDE assay never exceeded 2% (v/v). All assays were performed in quadruplicate.
Results
Cloning and Sequencing Analysis of Human PDE8A cDNA
By searching EST data base (8, 9) with the catalytic domains of various phosphodiesterases, we identified an EST sequences (GenBank accession number N79954) potentially encoding a novel phosphodiesterase. The deduced amino acid sequence of N79954 showed relative high homology (20-40%) to the conserved region in the catalytic domain of phosphodiesterase (data not shown). To determine the full-length cDNA sequence, we carried out 5' and 3' RACE (11). Because the EST sequence N79954 was originally derived from a human brain cDNA library, a similar library commercially prepared for RACE-PCR was used for the reaction. The full-length cDNA is 3,504 base pairs in length and contains a long open reading frame starting from the first methionine codon and encoding a polypeptide of a total 582 amino acids with a calculated molecular weight of 66,012 (Fig. 1)

The nucleotide sequence around the first methionine codon conforms well to the consensus sequence of the eukaryotic translational initiation site (14). The 3' noncoding region contains a typical AATAAA polyadenylation signal and four ATTTA motifs for rapid mRNA degradation.
The predicted protein shows significant homology to other PDEs in the catalytic domain. The amino acid identity of PDE8A is most closely related to the cAMP-specific PDE (39%), and the lowest to the photoreceptor PDE (23%) within the catalytic domain. The homology within this region between members of the known PDE families varies between 80-90%, and is not limited to the region, but extends throughout most of the coding region. However, the amino-terminal domain of PDE8A shows little homology to any known PDE families (data not shown). Thus, it appears that PDE8A is a novel isozyme that is most closely related to the cAMP-specific PDEs of family IV, but is not a member of this family.
Cloning and Sequence Analysis of Human PDE8B cDNA
Using the bioinformatic approach,, we also identified an EST sequence (GenBank accession number AA190710) homologous to the catalytic domain of PDE4 but was not identical to any known PDE (fig. 2)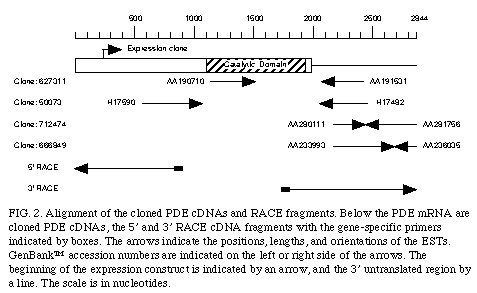
. This EST sequence was a 5' read sequence of human cDNA clone 627311 and the 3' read sequence of this clone is registered in the EST database as AA191531. Using the sequence of AA191531 we performed a secondary EST database search and isolated several homologous EST sequences (GenBank accession number H17482, AA28011, and AA233993). The clones corresponding to these ESTs were 50073, 712474, and 666849, respectively, and the opposite end sequences of these clones were also identified as H17590, AA281756, and AA236035, respectively (Fig. 2)
. These four clones were purchased from I.M.A.G.E. consortium and entirely sequenced. Based on this information, we carried out 5' and 3' RACE (11). As shown in Figure 2A, assembly of the clones and the RACE-PCR sequences gives the 2,844 nt. cDNA sequence that contains a long open reading frame encoding a polypeptide of 659 amino acids.
The nucleotide sequence around the third methionine codon (amino acid 76) in the open reading frame conforms well to the Kozak consensus sequence of the eukaryotic translational initiation site (14). While the nucleotide sequence around the first and second methionines (amino acid 13, 75) do not match the consensus sequence. Because the open reading frame continues to the beginning of the clone, it is not certain whether the sequence in Figure 3 
contains a complete coding sequence. The 3' noncoding region contains no typical polyadenylation signal (AATAAA), however, it does contain the alternative sequence (ATTAAA) 28 nt. upstream of the polyA tail. One ATTTA motif for rapid mRNA degradation is also found in the 3' noncoding region.
Homology searches of data bases revealed that this predicted protein is most similar to PDE8A, a recently identified isozyme of the new PDE family. The amino acid identity between this isozyme and PDE8A is 80% in the catalytic domain and 70% in the entire coding region (fig. 4)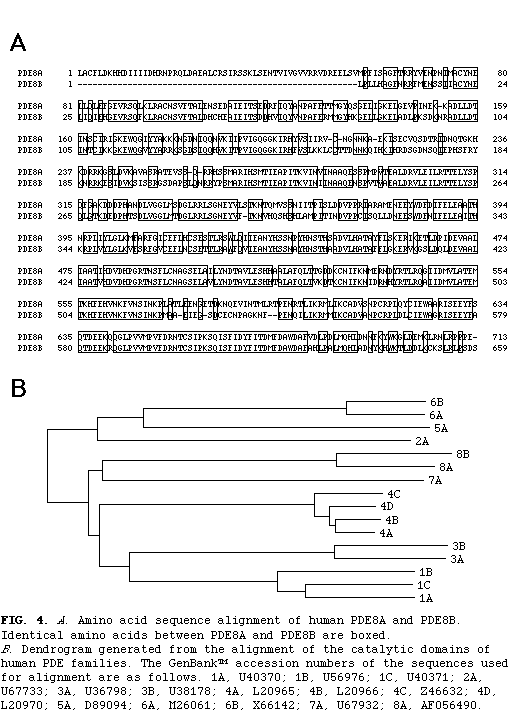
. Thus, it appears that this isozyme is a new member of the PDE8 family and we have designated it PDE8B.
Tissue Distribution of PDE8B
To examine the tissue distribution of the mRNA of PDE8A and PDE8B, Northern blot analysis were performed. The mRNA of PDE8A was found to be expressed ubiquitously in human tissues as a 4.2 kb transcript (Fig. 5)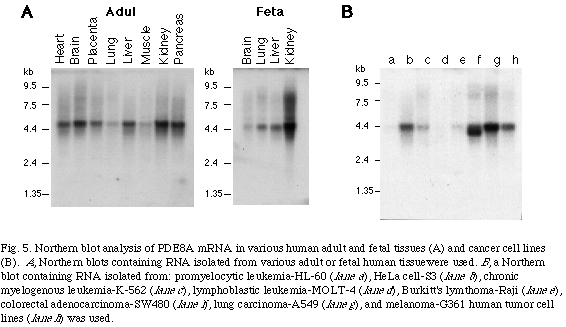
. Analysis of expression in various human transformed cells revealed that the mRNA was highly expressed in HeLa cell, lung carcinoma cell and melanoma cell lines in contrast to its lower or not detectable level of expression in human peripheral blood leukocytes. In addition, a truncated transcript (3.7 kb) was found to be highly expressed specifically in the colon adenocarcinoma cell line.
As shown in Figure 6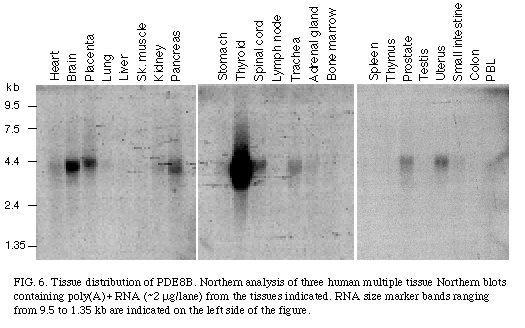
, the mRNA of PDE8B was found to be expressed as a 4.2 kb transcript and its expression was found to be specific to some tissues with the highest level in the thyroid gland, and with some expression in brain, spinal cord, and placenta.
Characterization of the Recombinant PDE8B
To characterize the PDE8B isozyme, the carboxyl-terminal 584 amino acids of PDE8B, generated from the third ATG codon, was expressed in E. coli and PDE activity was measured in bacterial lysates. Extracts from host expressing PDE8B exhibited up to 8-fold increases in cAMP PDE activity compared to E.coli extracts of cells carrying only the pET32 vector without insert (144.75 +- 6.58 vs. 18.06 +- 1.04 pmol/min/mg protein). No cGMP hydrolysis was detectable at concentrations up to 100 uM cGMP.
To examine whether the cAMP hydrolyzing activity of PDE8B might be regulated by cGMP as PDE2 or PDE3 family, cGMP was added. As shown in Figure 7 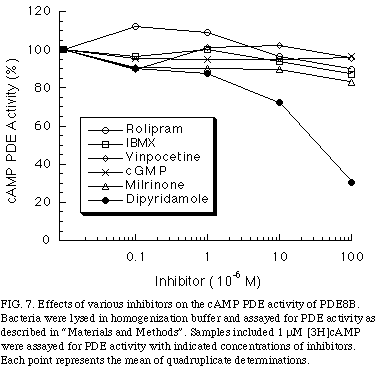
, concentrations of cGMP up to 100 uM had no effect on cAMP hydrolysis of PDE8B.
In addition, to determine whether PDE8B is pharmacologically related to other PDE families, we tested whether its PDE activity was sensitive to competitive inhibitors. The inhibitors used were vinpocetine; a PDE1 inhibitor, milrinone; a PDE3 inhibitor, rolipram; a PDE4 inhibitor, and dipyridamole, a PDE5, 6 ,8 inhibitor. As shown in Figure 5, cAMP PDE activity of PDE8B was not inhibited by any of these inhibitors, except dipyridamole, which had an IC50 of 40 uM. Moreover, PDE8B was insensitive to the non-specific inhibitor IBMX, a property that distinguishes the PDE8 family from the other known cAMP-specific PDEs, was observed.
Discussion and Conclusion
We cloned human cDNAs encoding two novel PDE isozymes, PDE8A and PDE8B. The amino acids in the catalytic domain between these two isozymes are highly conserved with a 78% identity and 87% similarity. This region of the protein contains the amino acids involved in cyclic nucleotide hydrolysis and is conserved among all known mammalian PDEs (6). In addition, the regions amino-terminal to the catalytic domain are also conserved in these two isozymes with a 65% identity and 83% similarity. This region is thought to be the regulatory domain and differs among PDE families but similar in the same family with a similarity ranging from 70 to 90%. Thus, these two isozymes are members of the new PDE8 family based on the sequence similarity.
The failure of PDE8B to hydrolyze cGMP or cGMP to modulate cAMP PDE activity of PDE8B suggest that this PDE is different from cGMP-PDEs, including PDE1, 2, 3, 5, and 6. In addition, the insensitivity of the recombinant PDE8B to various compounds including vinpocetine, milrinone, and rolipram indicates PDE8B is distinct from PDE1, 3, and 4 families. Finally, the ineffectiveness of IBMX to inhibit PDE8B clearly demonstrates that it is different from any known cAMP-specific PDE families including PDE4 and 7 (15, 16), but consistent with the pharmacological characteristic of the PDE8 family (3).
The most significant differences between PDE8B and PDE8A are tissue distribution. PDE8B is only expressed in specific tissues, especially the thyroid gland, while PDE8A is widely expressed. Several types of PDE families such as PDE1, PDE3, and PDE4 have been identified in the thyroid gland. However, these isozymes are widely expressed in a variety of tissues and are most abundant in other tissues such as brain or testis. To date, no other isozymes of PDEs have been found specifically and abundantly in the thyroid gland. High levels of specific PDE isozyme expression are often associated with specific functions of the region, such as the cGMP-specific PDE (PDE6) for visual transduction cascade in the retina (17) and the cGMP-stimulated PDE for the aldosterone production in the adrenal cortex (18). Although the relative abundancy of PDE8B to other PDE family in thyroid gland and the biological role of PDE8B is not presently understood, PDE8B may play an important role in a specific signal transduction pathway in the thyroid gland.
References
- Houslay, M. D., and Milligan, G. (1997) Trends in Biochem. Sci. 22(6), 217-224.
- Beavo, J. A. (1995) Physiol. Rev. 75(4), 725-748.
- Fisher, D. A., Smith, J. F., Pillar, J. S., St. Denis, S. H., and Cheng, J. B. (1998) Biochem. Biophys. Res. Commun. 246, 570-577.
- Fisher, D. A., Smith, J. F., Pillar, J. S., St. Denis, S. H., and Cheng, J. B. (1998) J. Biol. Chem. 273(25), 15559-15564.
- Soderling, S. H., Bayuga, S. J., and Beavo, J. A. (1998) J. Biol. Chem. 273(25), 15553-15558.
- Charbonneau, H. (1990) in Cyclic Nucleotide Phosphodiesterases: Structure, Regulation And Drug Action. Vol. 2, pp. 267-296, John Wiley & Sons, Ltd., Chichester.
- Manganiello, V. C., Murata, T., Taira, M., Belfrage, P., and Degerman, E. (1995) Arch. Biochem. Biophys. 322(1), 1-13.
- Boguski, M. S., Lowe, T. M., and Tolstoshev, C. M. (1993) Nat. Genet. 4(4), 332-3333.
- Boguski, M. S., Tolstoshev, C. M., and Bassett, D., Jr. (1994) Science 265(5181), 1993-1994.
- Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990) J. Mol. Biol. 215(3), 403-410.
- Frohman, M. A. (1993) Methods Enzymol. 218, 340-356.
- Gyapay, G., Schmitt, K., Fizames, C., Jones, H., Vega-Czarny, N., Spillett, D., Muselet, D., Prud'Homme, J. F., Dib, C., Auffray, C., Morissette, J., Weissenbach, J., and Goodfellow, P. N. (1996) Hum. Mol. Genet. 5(3), 339-346.
- Mukai, J., Asai, T., Naka, M., and Tanaka, T. (1994) Br. J. Pharmacol. 111(2), 389-390.
- Kozak, M. (1986) Cell 44(2), 283-292.
- Jin, S. L., Swinnen, J. V., and Conti, M. (1992) J. Biol. Chem. 267(26), 18929-18939.
- Michaeli, T., Bloom, T. J., Martins, T., Loughney, K., Ferguson, K., Riggs, M., Rodgers, L., Beavo, J. A., and Wigler, M. (1993) J. Biol. Chem. 268(17), 12925-12932.
- Fung, B. K., Hurley, J. B., and Stryer, L. (1981) Proc. Nat. Acad. Sci. U.S.A. 78(1), 152-156.
- MacFarland, R. T., Zelus, B. D., and Beavo, J. A. (1991) J. Biol. Chem. 266(1), 136-142.
| Discussion Board | Previous Page | Your Poster Session |