Ophthalmology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Autosomal Dominant Retinitis Pigmentosa(ADRP) ultimately leads to blindness through progressive degeneration of photoreceptor cells. The majority of ADRP cases are caused by mutations in the rhodopsin gene (1). Our ultimate goal is to develop a therapeutical tool that can delay the onset and slow down the progression of the disease. Our lab has demonstrated that tiplex forming oligonucleotides(TFOs) (2) can bind to special purine-rich regions of the rhodopsin gene in vitro.
To be able to study the effect of the TFOs in vivo, we need to establish a cell line that stably expresses rhodopsin. Because all sites that bind TFOs with high affinity were identified in the introns (3), we need to introduce the entire genomic copy into cells.
Rhodopsin is a 7 helix transmembrane protein, that contributes to 90% of total proteins in the rod cells of the retina. Class II. and III. rhodopsin mutations clog the ER, preventing the wild type protein from getting to the surface (4). In order to monitor the distribution of the protein inside the cell we created a "visible rhodopsin" construct by fusing green fluorescent protein (GFP) to it. We targeted this construct along with the native rhodopsin gene (no GFP) and the bovine cDNA into different cell lines and characterized some clones.
Materials and Methods
We constructed three different rhodopsin plasmids, in which the transcription of the gene is driven by the strong CMV promoter and which contain a neomycin resistance gene for selection of integrants. The human genomic rhodopsin-GFP fusion plasmid was made by eliminating the stop codon from the end of the rhodopsin gene and allowing continuous "in frame" reading through GFP.
Cells were transfected with Fugene 6 (Boehringer Mannheim), which is a lipid based but non-liposomal transfection reagent that gives high levels for transinet transfection and good rates for random integration. Cells were ~50% confluent at the time of transfection. For each 100mm tissue culture dish 6 ug plasmid was used with 20 ul Fugene 6. Cells were assayed 24 hours later for transient transfection. Stable clones were selected in medium containing 400 ug/ml G418 for 10-14 days.
The anti-rhodopsin antibody ("R2-15N" , generously provided by P. Hargrave ) used in the immunostaining and Western blot experiments is monoclonal and was originally generated against the N-terminal region of bovine rhodopsin (5). The corresponding N-terminal region in humans has identical aminoacid sequence to bovine rhodopsin, therefore we assumed that this antibody would be suitable for detection of the human protein.
The GFP antibody (Clontech) used for Western blots is a polyclonal antibody against synthetic GFP. Secondary antibodies used for immunostaining were labelled with Texas Red, for Western blots HRP-conjugated secondary antibodies were used.
Results
We constructed three different rhodopsin plasmids (Figure 1).
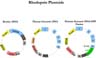 Click to enlarge
Fig.1: Rhodopsin plasmids
Click to enlarge
Fig.1: Rhodopsin plasmids
Equal amounts of these plasmids were transfected into several different cell lines: HT-1080, HeLa, 293, CHO. The karyotype of HT-1080 cells is closest to the normal human diploid conditions, therefore we focused on testing these cell lines. Immunostaining of transiently transfected cells show that the expression from the rhodopsin cDNA construct is higher than from the two other plasmids carrying the genomic rhodopsin cDNA (Figure 2).
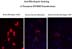 Click to enlarge
Fig.2: Transient rhodopsin expression
Click to enlarge
Fig.2: Transient rhodopsin expression
Cells transiently expressing the rhodopsin-GFP fusion protein gave positive results both with anti-rhodopsin immmunostaining and emission of green fluorescence, suggesting that the fusion protein is indeed being produced (Figure 3).
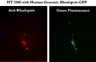 Click to enlarge
Fig.3: Dual fluorescence of a cell expressing rhodopsin-GFP
Click to enlarge
Fig.3: Dual fluorescence of a cell expressing rhodopsin-GFP
Using the neomycin resistance cassette we selected for stable integrants and tested several cell lines for the presence of rhodopsin.
Southern analysis and rt-PCR data ( not shown) suggest that some of the cell lines have integrated our construct and rhodopsin is being transcribed inside the cells. Western blots performed on these cell lines indicate that the protein is being made (Figure 4).
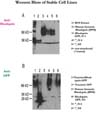 Click to enlarge
Fig.4: Western blot
Click to enlarge
Fig.4: Western blot
In Fig. 4 A we used anti-rhodopsin antibody to detect our protein. A cell line which has integrated the genomic rhodopsin construct (BP10 in lane 2) gives a 36 kDa band, the same size as the rhodopsin monomer in the rod outer segment (ROS) extract (in lane 1). The three cell lines that carry the rhodopsin-GFP construct ( N3, N4 and N5 in lanes 3-5) give higher molecular weight bands, whose size could correspond to the slower migrating fusion protein ( 36 kDa of rhodopsin + 29 kDa of GFP). The presence of these relatively high molecular weight bands in the corresponding lanes 4-6 of Fig. 4 B further support the idea, that the bands indicate the presence of the fusion protein. The anti-GFP antibody used for hybridization for this figure - as expected - does not give any signal with the BP10 cell line.
Anti-rhodopsin immunostaining of stable cell lines with genomic rhodopsin and rhodopsin-GFP give weak fluorescence, hardly distinguishable from background (data not shown). However stable cell lines expressing rhodopsin from the cDNA show intense staining. In vivo fluorescence of cells with rhodopsin-GFP (N3, N4, N5) is very faint (Figure 5).
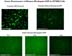 Click to enlarge
Fig.5: Green fluorescence of rhodopsin-GFP
Click to enlarge
Fig.5: Green fluorescence of rhodopsin-GFP
Only transiently transfected cells provide bright enough fluorescence to study intracellular distribution of rhodopsin (Figure 6).
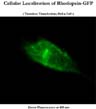 Click to enlarge
Fig. 6: Cellular localization of rhodopsin-GFP
Click to enlarge
Fig. 6: Cellular localization of rhodopsin-GFP
Discussion and Conclusion
We have successfully identified one HT-1080 cell line (BP10) that stably expresses rhodopsin and three cell lines that express the rhodopsin-GFP fusion protein (N3, N4, N5). However, the level of expression from the genomic rhodopsin constructs is much reduced related to that from the bovine cDNA construct. This could be due to transcription or processing differences between the cDNA and the genomic versions or to differences in the efficiency of antibody detection of bovine and human rhodopsin. The fact that green fluorescence from the stable rhodopsin-GFP fusion protein is also very marginal argues against the latter.
Green fluorescence from rhodopsin-GFP is much lower, than from GFP alone and brighter green cells that we might see 24 hrs post transfection disappear when we select with neomycin for cells that have integrated the plasmid into their genome. This could be due to multiple plasmids getting taken up by cells but only few of them actually integrating into the chromosome.
Even though the amount of protein produced from the genomic rhodopsin constructs is marginal, these cells can be used to detect changes in the rhodopsin transcription due to the binding of TFOs.
References
- Nathans, J (1992) Rhodopsin: structure, function and genetics. Biochemistry 31: 4923-4931.
- Vasquez, KM, Wilson, JH (1998) Triplex-directed modification of genes and gene activity. Trends Biochem. Sci. 23: 4-9.
- Perkins, DB, Wilson, JH, Wensel TG, Vasquez, KM (1998) Triplex targets in the human rhodopsin gene. Biochemistry 37: 11315-11322.
- Sung, C-H, Schneider, BG, Agarwal, N, Papermaster, DS (1991) Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. U.S.A. 88: 8840-8844.
- Rohlich, P, Adamus, G, McDowell, JH, hargrave, PA (1989) Binding pattern of anti-rhodopsin monoclonal antibodies to photoreceptor cells: an immunocytochemical study. Exp. Eye Res. 49: 999-1013.
| Discussion Board | Previous Page | Your Poster Session |