Invited Symposium: Signal Transduction in Endothelium: Mechano-Sensing, Ion Channels and Intracellular Calcium
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Cation channels which allow permeation of Na+ as well as K+, and to some extent also permeation of divalent cations, are present in in the plasma membrane of endothelial cells [1-5]. Endothelial non-selective cation channels are activated in response to stimulation of phospholipase C-coupled receptors or depletion of Ca2+ stores [1-3] and during oxidative stress [4,5]. The molecular nature and the exact role of these channels is still elusive. One family of membrane proteins that has been reported to constitute cation channels of variable ion selectivity is the Trp (Drosophila transient receptor potential gene product) protein family [6]. It appears likely that a variety of TRP channels with different activation and permeation properties are formed by heteromultimerization of Trp proteins. Recently, evidence has been presented for the expression of Trp homologues in vascular endothelium [7,8]. Here we present evidence that Trp homologues form non-selective cation channels of physiologic and pathophysiologic relevance in vascular endothelial cells.
Materials and Methods
Cell culture
Endothelial cells from human umbilical vein (HUVEC) and porcine aorta
(ECAP) as well as human embryonic kidney cells (HEK 293) were cultured
as described [8,9]. Oxidative stress was introduced by incubation of cells
in serum free medium containing 400 µM tBHP 60 minutes prior to experimentation.
RT-PCR
Total RNA was prepared from HUVEC, ECAP and HEK 293 cells
and RT-PCR experiments as well as Southern-blotting were performed as described
[8].
DNA constructs and cell transfection
Constructs used for expression were in the bicistronic expression vector
pIRES-EGFP (Clontech). NTRP3 consisted of a fragment corresponding to the
amino acids 1-302 of hTrp3 (U47050). CTRP3 consisted of a fragment comprising
the amino acids 721-848 of hTrp3. Endothelial cells were transiently transfected
using Superfect reagent (Qiagen).
Electrophysiology
Whole-cell currents were recorded with standard patch-clamp technique
as described [9]. The pipette solution contained : 110 mM K-gluconate,
10 mM KCl, 5 mM MgCl2, 10 mM HEPES, 10 mM BAPTA. The free Ca2+
concentration of the pipette solution was approximately 50 nM. The standard
bath solution contained : 137 mM NaCl, 5.4 mM KCl, 2 mM CaCl2,
15 mM HEPES, pH of all solutions was adjusted to 7.4 with NMDG (N-methyl-D-glucamine).
In some experiments rapid depletion of intracellular Ca2+ stores
was induced upon obtaining conventional whole cell configuration due to
the inclusion of 100 µM IP3 in the pipette solution. When
NaCl was omitted in the bath solution, osmolaritiy was kept constant by
addition of choline chloride.
Statistics
Averaged data are given as mean ± S.E.M. Statistical analysis
was performed using Student's t-test for unpaired values and differences
were considered statistically significant at P < 0.05.
Materials
Tissue culture media were from Gibco BRL (Vienna, Austria), all other
chemicals from Sigma Chemical Co. (Vienna, Austria).
Results
Vascular endothelial cells express Trp proteins
The presence of transcripts of trp genes in human and porcine endothelial
cells was tested with a RT-PCR strategy. HEK 293 cells which are known
to express various Trp isoforms [10] were used as reference. In HUVEC,
expression of Trp1 and Trp3 was tested with primers specific for the human
isoforms hTrp1 [11] and hTrp3 [12]. For analysis of Trp4 expression, primers
specific for a recently reported partial human sequence [12] as well as
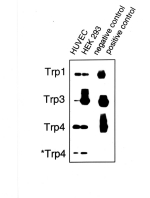
primers specific for mTrp4 [12] were used. Fig. 1 shows a Southern blot
hybridization of PCR products obtained with RNA from HUVEC and HEK 293,
HUVEC RNA which was not subjected to reverse transcription (negative
control) as well as with available target cDNA clones (hTrp1, hTrp3 and
mTrp4) as template (positive control). PCR products of the expected size of all three isoforms
were obtained with reverse transcribed RNA preparations from HUVEC and
HEK 293 cells but not in negative controls.
 Click here to enlarge Fig.1
Click here to enlarge Fig.1
Fig. 1: Expression of different Trp isoforms in HUVEC.
Trp genes (Trp 1, 3 and 4) were amplified by RT-PCR, blotted and probed with digoxygenin-labeled oligonucleotides internal to the PCR primers used. Two different sets of primers were used to amplify Trp4: one was to bind to mTrp4 (indicated as Trp4), the other one to hTrp4 (indicated as *Trp4). RT-PCR experiments were performed with total RNA from HUVEC and HEK293. Total RNA from HUVEC was subjected to PCR without preceeding reverse transcription as a negative control. Three available cDNA clones (hTrp1, hTrp3 and mTrp4) were used as PCR templates for a positive control.
The results indicate expression of three human Trp species in human umbilical vein endothelium. Similarily, RT-PCR products of the expected size (448 bp) were obtained from total RNA preparations of ECAP with primers specific for hTrp3, indicating the expression of a Trp3 isoform in porcine aortic endothelial cells (not shown).
Depletion of intracellular Ca2+ stores and oxidative stress are associated with activation of a non-selective cation conductance
Depletion of intracellular Ca2+ stores by dialyses of HUVEC
with IP3 plus the Ca2+ chelator BAPTA (10 mM) induced
a substantial increase in membrane conductance. The increase in inward
current at -80 mV was larger and more stable with Na+ (137 mM)
than with Ca2+ (10 mM) as charge carrier (not shown). Typical
membrane currents recorded in a Na+ -containing, Mg2+
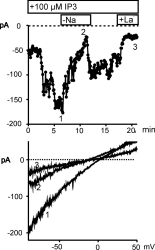
-free solution are shown in Fig. 2.
 Click here to enlarge Fig.2
Click here to enlarge Fig.2
Fig. 2: Ca2+ store depletion by IP3 induces a cation current
in HUVEC.
HUVEC were dialyzed with IP3 (100 ÁM) via the pipette solution starting with rupture of the patch at time 0. Upper panel: Time-course of membrane current at 80 mV is shown for a cell which was transiently transfected with GFP. Removal of extracellular Na+ and addition of La3+ (50 ÁM) is indicated. Lower panel: Current to voltage relationship derived at the time points indicated.
Fig. 2. shows the time course of the membrane current
recorded at -80 mV (upper panel). This current was dependent on extracellular Na+
blocked by 50µ M La3+ (N = 7). Lower panel of Fig. 2 illustrates
the current to voltage relationship of the IP3 -induced current
that reversed at about neutral potential. Removal of extracellular Na+
resulted in a reduction of current and shifted the reversal potential in
hyperpolarizing direction indicating that the current is carried for a
large part by Na+. The IP3 -induced cation conductance
was observed with high reproducibility (14 out of 15 cells).
ECAP exhibited very small IP3 -induced membrane currents.
However, a current reminiscent of IP3 -induced membrane currents
was observed after prolonged exposure (60 min) of cells to the lipophilic
oxidant tBHP (400 µ M) Oxidative stress resulted in a dramatic increase
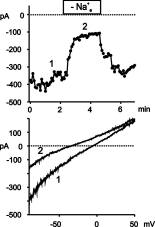
in membrane conductance of ECAP. As shown in Fig. 3., large inward currents
were detectable in tBHP-treated ECAP at negative membrane potentials (-80
mV).
 Click here to enlarge Fig.3
Click here to enlarge Fig.3
Fig. 3: tBHP activates a cation conductance in ECAP.
Upper panel: Time course of membrane currents at -80 mV recorded from a cell after exposure to tBHP (400 ÁM, 60 min). Removal of extracellular Na+ [Na+e] leads to reversible shift in the current to voltage relationship. Lower panel: current to voltage relationships derived at the time points indicated in the upper panel.
Removal of extracellular Na+ reduced the inward currents at -80 mV due to a shift of the zero potential to more negative values indicating a significant contribution of Na+ to the tBHP-induced current. Similarily to the IP3-induced current of HUVEC, the oxidant-induced current was completely blocked by 50 µM La3+ (data not shown).
Endothelial non-selective membrane conductances are eliminated by expression of the N-terminal domain of hTrp3
The N-terminal domain (residues 1-302) of hTrp3 was cloned into the
vector pIRES-EGFP which allows for simultaneous expression of proteins
and the marker protein GFP. Endothelial cells were transiently transfected
with N-TRP in pIRES-EGFP. The rational for this strategy is the observation
that N-terminal fragments of Trp proteins exert a dominant negative effect
on Trp channel function [13]. Control experiments were performed in cells
transfected pIRES-EGFP only. Some experiments were performed with cells
transfected with full length hTrp3 which is expected to interact with endogenous
Trp proteins without preventing the formation of functional Trp oligomers.
Alternatively, cells were transfected with C-TRP (residues 721-848 of hTrp3).
HUVEC transfected to express GFP only, responded to intracellular administration
of IP3 with a clear increase in the membrane current recorded
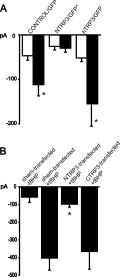
at -80 mV (N=14). As illustrated in Fig. 4A, the inward current recorded
at -80 mV in N-TRP-transfected HUVEC remained stable during dialysis with
IP3 (100 µ M; 10 - 20 min recording time; N = 15). Cells
transfected with hTrp3 responded to intracellular administration of IP3 in a manner
similar to GFP-transfected controls, exhibiting even slightly larger increases
in membrane conductance.
 Click here to enlarge Fig.4
Click here to enlarge Fig.4
Fig. 4: Modification of endothelial cation currents by expression of TRP proteins.
A. Mean inward currents (at 80 mV), recorded
initially (open columns) and 4-6 min after obtaining the whole cell configuration with IP3-containing pipette solution (filled columns) in cells transfected with GFP only (control/GFP; N=14), or NTRP3 plus GFP by transfection of NTRP3 in pcDNA3-pIRES-EGFP (NTRP3/GFP*; N=5) or hTrp3 plus GFP (hTrp3/GFP; N=5).
B. Expression of NTRP3 but not CTRP3 suppresses tBHP-induced cation conductance. Membrane currents at -80 mV in sham-transfected endothelial cells without (-tBHP; N=4) or with pretreatment of cells with the oxidant (+tBHP; N=12), NTRP3-transfected (+tBHP; N=14) and CTRP3-transfected (+tBHP; N=5).
Mean values S.E.M.;* indicates significant differences versus initial current value (A) or versus control + tBHP (B) respectively.
Transfection of HUVEC with N-TRP clearly suppressed the IP3/store depletion-induced
cation conductance. It is of note that other characteristic membrane conductances
such as the inwardly rectifying K+ conductance appeared unaffected
(not shown).
To test whether Trp proteins are involved in the tBHP-induced cation
conductance, ECAP were transiently transfected to express two different
cytosolic domains of hTrp3: either i) the amino terminal domain (amino acid residues 1-302,
referred to as N-TRP), or ii) the carboxy terminal domain (amino acid residues 721-848,
referred to as C-TRP). The transfection procedure itself did not affect
the response of ECAP to tBHP (sham transfection) as shown in Fig. 4B. Similarly,
ECAP expressing only GFP due to transfection with pIRES-EGFP displayed
no modification of the response to tBHP (not shown). In clear contrast,
transfection of ECAP with the N-TRP construct resulted in a significant
suppression of the tBHP-induced current at -80 mV. By contrast, transfection of ECAP
with C-TRP failed to suppress the inward currents induced by tBHP.
Discussion and Conclusion
In summary, our results suggest that homologues of the Drosophila
transient receptor potential (Trp) gene product contribute to two distinct
non-selective cation conductance of vascular endothelial cells, i.e. an
IP3 -induced cation conductance which may serve physiologic
activation of endothelial cells and a rather pathophysiologically relevant
cation conductance which is activated by oxidative stress. Our conclusion
is based on the observation that expression of a N-terminal fragment of
a Trp protein (N-TRP) that is known to exert a dominant negative effect
on Trp channel function, eliminates both cation conductances. The well
documented ability of N-TRP to bind to the N-terminal domain of other Trp
proteins [13] is expected to result in an interaction of N-TRP with endogenous
Trp proteins and consequently in suppression of the assembly of functional
Trp oligomers. Our results favour the idea of an involvement of Trp proteins
in the phenomena of IP3 - and oxidant-induced cation conductances
of endothelial cells. Expression of complete hTrp3 or of
a C-terminal fragment of hTrp3 (C-TRP) failed to affect non-selective conductances
in endothelial cells. Expressed hTrp3 is expected to associate with endogenous
Trps without essential impairment of channel function and expression of
a C-terminal fragment is not expected to interfere with assembly of endogenous
Trp channels. Despite the apparent differences in the mechanism of activation,
IP3- and oxidant-induced cation conductances of endothelial cells exhibited strikingly
similar properties, i.e. sensitivity to inhibition by N-TRP, a rather high
permeability for Na+ ions and relatively high sensitivity to
block by La3+. Activation of cation channels by intracellular
administration of IP3 in the presence of the Ca2+
chelator BAPTA may involve either a direct interaction of IP3
with the channel or a mechanism related to depletion of intracellular Ca2+
stores. In particular the latter mechanism has been implicated in activation
of specific isoforms of Trp [6]. Our present knowledge on Trp proteins suggest
an important role of some species in hormone-regulated Ca2+
but also of monovalent cation conductances [6,14,15]. Expression of some
members of the Trp protein family such as Trp1 and 3 gave rise to poorly
selective cation conductances which allow for large Na+ currents
[15] similar to that observed in endothelial cells upon exposure to oxidative
stress. Oxidative stress by itself does not release Ca2+ from
intracellular stores [16]. Thus, it appears unlikely that oxidative stress
activates Trp channels by depletion of Ca2+ stores.On the other hand, direct redox sensitivity
of Trp channels has, to our knowledge, not yet been studied. Nonetheless,
a mechanism of activation independent of intracellular Ca2+
handling such as oxidation of critical sulfhydryl groups due to accumulation
of oxidized glutathione [4,5] (see below Fig.5) may well be considered for Trp channels.
Non-selective Trp channels may, to some extent, contribute to physiologic
Ca2+ homeostasis. Excessive activation of such Trp channels, however,
is expected to result in cellular Na+ loading and membrane depolarization
as observed in oxidative stress. Membrane depolarization is known to suppress Ca+ signalling in endothelial cells. The consequences of Na+ loading on the other hand are so far barely understood. Changes in the intracellular Na+ concentration may well interfere with cellular Ca2+ signals and regulation of endothelial functions due to control of subcellular Ca+ gradients via Na+/Ca2+ exchange [17].
So far up to five trp genes were detected
in vascular endothelial cells [4; 5]. Formation of various heteromultimeric
Trp complexes [14] may well give rise to a functional diversity among the
Trp channels in specific tissues. Thus, it appears conceivable to speculate
that Trp heteromultimers are the molecular basis more than one cation conductance in
endothelial cells. One group may serve hormonal control of Ca2+
homeostasis while another group may play a role in pathophysiologic situations
such as oxidative stress as (Fig. 5).
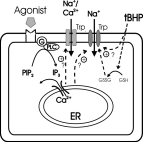 Click here to enlarge Fig.5
Click here to enlarge Fig.5
Fig. 5: Hypothetical functions of endothelial TRPs.
TRP proteins may be involved in multiple ion conductances of endothelial cells. These TRP-mediated conductances include a store-operated Ca2+ entry pathway and a redox-activated Na+
conductance. Abbreviations used: ER: endoplasmatic reticulum; PLC: phospholipase C; PIP2: phosphatidylinositolbisphosphate; IP3: inositoltrisphosphat; GSH: glutathione; GSSG: oxidized
glutathione
In summary, we suggest a central physiologic as well as pathophysiologic role
of non-selective cation channels formed by Trp heteromultimers in endothelial
cells. Endothelial Trp proteins may on the one hand serve hormonal control
of Na+ and Ca2+ homeostasis and in addition determine
cellular redox sensitivity.
The authors wish to thank Dr. M. Poteser for his efforts preparing this manuscript and Dr. X. Zhu for kindly providing TRP cDNA-clones.
The work was supported by the Austrian Research Funds, SFB Biomembranes F708 and F715 as well as P12667.
References
- Nilius B (1990) Permeation properties of a non-selective cation channel in human vascular endothelial cells. Pflugers Arch. 416: 609-611.
- Nilius B, Schwartz G, Oike M, Droogmanns G (1993) Histamine-activated, non-selective cation currents and Ca2+ transients in endothelial cells from human umbilical vein. Pflugers Arch 424: 285-293.
- Yamamoto Y, Chen G, Miwa K, Suzuki H (1992) Permeability and Mg2+ blockade of histamine-operated cation channel in endothelial cells of rat intrapulmonary artery. J Physiol (Lond). 450:395-408.
- Koliwad SK, Kunze DL, Elliott SJ (1996a) Oxidant stress activates a non- selective cation channel responsible for membrane depolarization in calf vascular endothelial cells. J Physiol 491: 1-12
- Koliwad SK, Kunze DL, Elliott SJ (1996b) Oxidized glutathione mediates cation channel activation in calf vascular endothelial cells during oxidant stress. J Physiol 495:37-49
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M (1996) On the molecular basis and regulation of cellular capacitative Ca2+ entry: roles of TRP proteins. Proc Natl Acad Sci USA 93:15195-15202
- Chang AS, Chang SM, Garcia RL, Schilling WP (1997) Concomitant and hormonally regulated expression of trp genes in bovine aortic endothelial cells. FEBS Lett. 415:335-340
- Groschner K, Hingel S, Lintschinger B, Balzer M, Romanin C, Zhu X, Schreibmayer W (1998) TRP proteins form store-operated cation channels in human vascular endothelial cells. FEBS Lett 437: 101-106
- Groschner K, Graier WF, Kukovetz WR (1994) Histamine induces K+, Ca2+, and Cl-currents in human vascular endothelial cells. Role of ionic currents in stimulation of nitric oxide biosynthesis. Circ Res 75:304-314
- Garcia RL, Schilling WP (1997) Differential expression of mammalian TRP 10 homologues across tissues and cell lines. Biochem Biophys Res Comm 239:279-283
- Zhu, X., Chu, P.B., Peyton, M., and Birnbaumer, L (1995) Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett 373:193-198.
- Zhu X, Peyton M, Birnbaumer L (1996) TRP, a novel mammalian gene essential for agonist-activated capacitative Ca2+ entry. Cell 85:661-671
- Xu XS, Li H, Guggino WB, Montell C (1997) Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell 89:1155-1164
- Philipp S, Cavali* A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V (1996) A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J 15:6166- 6171.
- Hurst RS, Zhu M, Boulay G, Birnbaumer L, Stefani E (1998) Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Lett. 422: 333-338
- Wesson DE, Elliott SJ (1994) Xanthine oxidase inhibits transmembrane signal transduction in vascular endothelial cells. J Pharmacol Exp Ther. 270:1197-1207
- Graier WF, Paltauf-Doburzynska J, Hill B, Fleischhacker E, Hoebel B, Kostner GM, Sturek M (1998) Submaximal stimulation of porcine endothelial cells causes focal Ca2+ elevation beneath the cell membrane. J Physiol Lond 506.1:109-125
| Discussion Board | Previous Page | Your Symposium |