Neuropharmacology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The acoustic startle response (ASR) is a series of facial and skeletal muscle contractions in response to a sudden and/or intense noise. The amplitude of the acoustic startle response is markedly decreased when a weak stimulus (a prepulse) precedes a startle-eliciting stimulus. This phenomenon is known as prepulse inhibition (PPI). Patients with schizophrenia demonstrate a reduced PPI compared to normal subjects: they show less inhibition (Braff et al., 1992; Cadenhead et al., 1993). PPI is also attenuated in rats systematically administered direct dopamine agonists, such as apomorphine, or indirect dopamine agonists such as amphetamine and cocaine (Swerdlow et al., 1986; Mansbach et al., 1988; Harty and Davis, 1985). Thus, it is plausible that the reduction in PPI in schizophrenia is due to increased dopaminergic transmission in these patients. In recent unpublished studies from our laboratory, the effects of a variety of direct and indirect dopamine agonists on PPI in rats were tested with a range of time intervals between the prepulse and startle pulse, known as stimulus onset asynchronies (SOAs). While some differences among dopamine agonists were observed in terms of reductions in PPI, all agonists were found to decrease the SOA at which the maximal inhibition occurred. It is possible that dopaminergic effects on PPI in rats are due to a shift to the left of the SOA-PPI function, and that other neurotransmitter systems contribute to the PPI attenuating effects of drugs such as amphetamine.
Some reports have suggested that the serotonergic system may contribute to the modulation of PPI. Sipes and Geyer (1993) found that the 5-HT2 agonist, 2,5-dimethoxy-4-iodoamphetamine (DOI), disrupted PPI, and that this effect was reversible by ketanserin, a selective 5-HT2 antagonist. Selective 5-HT1A agonists, such as 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT), buspirone and ipsapirone, have also been found to disrupt PPI in rats (Rigdon and Weatherspoon, 1992; Sipes and Geyer, 1995).
In summary, psychomotor stimulants such as amphetamine appear to have two effects on PPI: decreasing the SOA at which maximal PPI occurs and decreasing the maximal PPI produced across SOAs. These two effects may be independent, as some drugs produce one effect without the other. It is possible that one effect is dopaminergic and the other serotonergic. This study, therefore, aims to examine the effects of selective blockade of D2-like and 5-HT2 receptors on both the decrease in maximal PPI and the shortening of the SOA at which maximal PPI occurs produced by amphetamine administered to rats.
Materials and Methods
Seventy-two male Wistar rats from the Animal Resource Center of Western Australia served as subjects. The rats, weighing between 258-355 g on arrival, were housed in the colony room, a temperature-controlled animal-housing facility on a 12 hour light/dark cycle (lights on 0700-1900), for eight days before beginning the experiment. Testing was conducted in eight standard Med Associates startle apparatus in a fan-ventilated and sound-attenuated chamber lit with a dim red light. All drugs were dissolved in a saline vehicle and injected subcutaneously with an injection volume of 1 ml/kg. Doses were 3 mg/kg (+)amphetamine sulfate, 0.05 mg/kg raclopride, 2.0 mg/kg ketanserin; all doses are expressed as the weight of the salt.
Procedures
Animals were randomly assigned to one of six groups: vehicle/vehicle (n=12), vehicle/amphetamine (n=13), ketanserin/vehicle (n=11), ketanserin/amphetamine (n=11), raclopride/vehicle (n=13), and raclopride/amphetamine (n=12). Each animal was tested three times, with three to four days between each session. The first test was a baseline non-drug test in which the rats were injected with a saline solution (1 ml/kg). The second two tests were drug tests. Animals were then placed in the startle chambers for a 10-minute acclimatisation period with a background noise of 65 dB. Fourteen trials were presented in random order in each block: (i) a null trial, with no startle or prepulse stimulus (only the background noise), (ii) two pulse-only trials, and (iii) 11 prepulse + pulse trials. The pulse-only stimulus was a 40 ms broad spectrum white noise at 105 dB with a rise/fall time of 0 ms. The prepulse stimulus was an 8 ms, 70 dB white noise with a rise/fall time of 1 ms. The interval between the onset of the prepulse stimulus and the onset of the pulse stimulus (SOA) varied in 10 ms intervals from 10 ms to 100 ms, with an additional 120 ms SOA trial included. The inter-trial interval varied randomly between 10-20 sec. In all, 12 blocks were conducted, resulting in 168 trials per animal.
Results
There were no significant differences in startle responses or in PPI among the groups on the baseline (vehicle only) test (data not shown). Responses on the two drug test day were averaged to provide a single measure under each trial condition. As can be seen in Figure 1, amphetamine increased motor activity on nonstimulus (null) trials, and this effect was blocked by raclopride but not ketanserin.
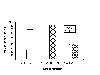 Click to enlarge
Fig. 1: Motor activity levels as measured by activity on null trials. The error bar represents critical difference values from the multiple F-test.
Click to enlarge
Fig. 1: Motor activity levels as measured by activity on null trials. The error bar represents critical difference values from the multiple F-test.
These effects were significant, as indicated by the multiple F-test for individual comparisons. In addition, a two-way ANOVA (amphetamine [vehicle vs amphetamine] and antagonist [vehicle, ketanserin or raclopride] revealed a significant main effect of amphetamine [F(1,65) = 52.63, p<.001] and of antagonist [F(2,65) = 3.14, p < .05]. As illustrated in Figure 2, mean startle amplitudes on startle-only trials were significantly increased by the administration of amphetamine [F(1,65) = 81.31, p <.001]. This effect was not blocked by either ketanserin or raclopride. However, a main effect of the antagonist was also found [F(2,65) = 3.77, p < .05]. Planned comparisons indicated that both ketanserin and raclopride significantly decreased mean startle amplitude after the saline injection, but only raclopride reduced startle amplitude after the administration of amphetamine.
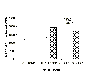 Click to enlarge
Fig. 2: Mean startle amplitude as measured by activity on startle-only trials. The error bar represents critical difference values from the multiple F-tests. An asterisk (*) represents a significant difference from the veh + sal group. A cross (+) indicates a significant difference from the veh + amp group.
Click to enlarge
Fig. 2: Mean startle amplitude as measured by activity on startle-only trials. The error bar represents critical difference values from the multiple F-tests. An asterisk (*) represents a significant difference from the veh + sal group. A cross (+) indicates a significant difference from the veh + amp group.
As depicted in Figure 3, amphetamine significantly decreased maximum PPI [F(1,65) =12.58, p < .001]. There was a main effect of the antagonists [F(2,65) = 4.72, p = .012]. Planned comparisons indicated that ketanserin had no significant effect on maximum inhibition (see Figure 3). While raclopride tended to inhibit startle after the administration of saline, the effect was only significant by individual comparisons after amphetamine.
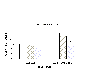 Click to enlarge
Fig. 3: The effect of the control and drug groups on maximum inhibition across all SOAs. The asterisk (*) represents a significant difference between the veh + amp group and the rac + amp group. Critical differences were calculated from the multiple F-test.
Click to enlarge
Fig. 3: The effect of the control and drug groups on maximum inhibition across all SOAs. The asterisk (*) represents a significant difference between the veh + amp group and the rac + amp group. Critical differences were calculated from the multiple F-test.
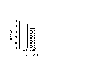
A main effect of amphetamine was found [F(1,65) = 16.05, p < .001], with amphetamine significantly reducing the SOA at which the maximum inhibition occurred from an overall mean SOA of 44 ms to 36 ms (see Figure 4). This shortening effect of amphetamine was not blocked by either ketanserin or raclopride [F(2,65) = 0.08, ns]. No amphetamine x antagonist interaction was found.
 Click to enlarge
Fig. 4: The effect of amphetamine on the SOA of maximum inhibition (n=36 per group). The error bar represents critical differences calculated from the multiple F-test.
Click to enlarge
Fig. 4: The effect of amphetamine on the SOA of maximum inhibition (n=36 per group). The error bar represents critical differences calculated from the multiple F-test.
Discussion and Conclusion
The present study examined the effects of blockade of D2-like and 5-HT2 receptors on rats treated with amphetamine, by measuring maximal PPI and the SOA of maximal PPI. It was predicted that the indirect dopamine agonist, amphetamine, would decrease the amount of PPI, as well as reduce the SOA at which maximal inhibition occurred. In line with this prediction, amphetamine was found to have a significant attenuating effect on PPI. This finding replicates the well-established literature on amphetamine-induced deficits in PPI (Druhan et al., 1998; Mansbach et al., 1988; Swerdlow et al., 1994). In further support of the experimental hypothesis, amphetamine was also found to significantly reduce the SOA at which maximum inhibition occurred, replicating the unpublished findings of this laboratory.
When administered alone, raclopride was found to reduce startle on startle-only and prepulse + pulse trials. This finding replicates the findings of Johansson and colleagues (1995) who found that PPI was facilitated by treatment with the D2 antagonists, haloperidol and raclopride. Raclopride blocked the effect of amphetamine on general motor activity (measured on null trials), as would be expected. Ketanserin was without effect on amphetamine-induced motor activity. Both raclopride and ketanserin reduced startle, but neither blocked the effect of amphetamine at increasing startle. Therefore, amphetamine's effects on increasing startle on startle-stimulus only trials does not appear to depend on either dopamine D2 or 5-HT2 receptors. Raclopride, but not ketanserin, blocked the effects of amphetamine on reducing maximum PPI. However, neither raclopride nor ketanserin blocked the effects of amphetamine on shifting the SOA of maximum PPI. This was somewhat surprising, as unpublished data from this laboratory indicate that two selective D2 agonists (+)PHNO and quinpirole shift the SOA of maximum PPI without reducing maximum PPI across SOAs, when PPI is defined as a proportion of the startle amplitudes on the startle-stimulus only trials (as opposed to difference scores).
Thus, amphetamine produces two independent effects on PPI. D2 receptors blocked by raclopride are necessary for the effect of amphetamine on maximum PPI across SOAs. As selective D2 agonists do not reduce maximum PPI when defined as a proportion of the startle amplitudes on the startle-stimulus only trials, it appears that D2 receptors are necessary but not sufficient for this effect. However, D2 receptors are not necessary for the shift to a shorter SOA at which maximum PPI occurs. As selective D2 receptor agonists can shift the SOA at which maximum PPI occurs, it appears that D2 receptors are sufficient, but not necessary, for this action. 5-HT2 receptors are not necessary for either effect of amphetamine.
It can be concluded that dopamine D2 receptors are involved in both effects on PPI, but via separate mechanisms.
References
- Braff, D, Grillon, C, & Geyer, M (1992) Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 49: 206-215.
- Cadenhead, K, Geyer, M, & Braff, D (1993) Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry 150: 1862-1867.
- Druhan, J, Geyer, M, & Valentino, R (1998) Lack of sensitisation to the effects of d-amphetamine and apomorphine on sensorimotor gating in rats. Psychopharmocology 135: 296-304
- Harty, P, & Davis, M (1985) Cocaine: Effects on acoustic startle and startle elicited electrically from the cochlear nucleus. Psychopharmacology 87: 396-399.
- Johansson, C, Jackson, D, Zhang, J, & Svensson, L (1995) Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: Effects of antipsychotics and other agents in rats. Pharmacol Biochemistry Behav 52: 649-654.
- Mansbach, R, Geyer, M, & Braff, D (1988) Dopaminergic stimulation disrupts sensorimotor gating in the rat. Neuropharmacol 33: 441-448.>
- Rigdon, G, & Weatherspoon, J (1992) 5-Hydroxytryptamine-sub (1a) receptor agonists block prepulse inhibition of acoustic startle reflex. J Pharmacol Exp Ther 263: 486-493.
- Sipes, T, & Geyer, M (1993) Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacol 33: 441-448.
- Sipes, T, & Geyer, M (1995) 8-OH-DPAT disruption of prepulse inhibition in rats: Reversal with (+)WAY 100,135 and localisation of site of action. Psychopharmacology 117: 41-48.
- Swerdlow, N, Braff, D, Geyer, M, & Koob, G (1986) Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biol Psychiatry 21: 23-33.
- Swerdlow, N, Braff, D, Taaid, A, & Geyer, M (1994) Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 51: 139-154.
| Discussion Board | Previous Page | Your Poster Session |