Neuropharmacology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
There is substantial evidence suggesting that adenosine is an important neuromodulator in the CNS. Adenosine has been shown to modulate transmitter release under physiological and pathophysiological conditions. The levels of extracellular adenosine in the brain are increased after anoxia and ischemia (Hagberg et al. 1987, Lloyd et al. 1988, Dux et al. 1990). Under these conditions, adenosine may act as an endogenous neuroprotectant, mainly by decreasing the release of excitatory amino acids (Dunwiddie and Hoffer 1980, Fastbom and Fredholm 1985), inhibiting the excitatory actions of glutamate (Siggins and Schubert 1981) and increasing cerebral blood flow (Winn et al 1981). Adenosine exerts its effects on neurotransmitter release mainly via A1 and A2 receptors and is known to influence potently the release of several neurotransmitters, such as acetylcholine, glutamate, noradrenaline, dopamine, serotonin and GABA (Review: Fredholm and Dunwiddie 1988).
Now we have investigated whether adenosine modulates the release of histamine in the anterior hypothalamus which contains a relatively high density of histaminergic nerve endings. The anterior hypothalamus of anaesthetized rats was superfused through a push-pull cannula either with drugs which influence extracellular adenosine concentration, or with ligands of adenosine receptors and the released histamine was determined in the superfusate by HPLC. In some experiments, extracellular adenosine outflow was also investigated.
Materials and Methods
Push-pull technique
Male rats (260-300 g body weight) were anaesthetized with urethane, their head fixed in a stereotaxic frame and a push-pull cannula (outer tubing: outer diameter 0.82 mm, inner tubing: outer diameter 0.25 mm) mounted on a microdrive was inserted stereotaxically through a hole in the skull into the anterior hypothalamic area (mm from bregma: A.P. -1.5, V. 8.0, L. 0.5).
The rat hypothalamus was superfused at a rate of 20 µl/min with artificial cerebrospinal fluid (CSF; mmol/l: NaCl 140, KCl 3.0, CaCl21.2, MgCl2 1.0, NaH2PO4 1.0, glucose 3.0, pH 7.2) or with drugs dissolved in CSF. The superfusate was collected continuously in time periods of 10 min, mixed with 1/10 volume of perchloric acid (0.1mol/l) and frozen at -20° C. The position of the cannula was verified in histologic slices. Histamine was determined by HPLC with fluorimetric detection (Prast et al. 1994). Adenosine was determined by HPLC combined with UV detection (Fischer et al. 1995).
The mean release rate in the two samples preceding superfusion with drugs was taken as 1, and data were expressed as relative values. Data are shown as means ± SEM of relative values. Statistical significance was calculated by Friedman's analysis of variance, followed by Wilcoxon's signed rank test.
Results

Fig.1
A: Superfusion of the hypothalamus for 20 min with the A1 adenosine receptor agonist N6-cyclopentyladenosine (CPA, 50 µmol/l) increased the release rate of histamine. B: The A1 receptor antagonist 8-cyclopentyl-1,3-dimethylxanthine (CPT, 50 µmol/l) decreased histamine release.
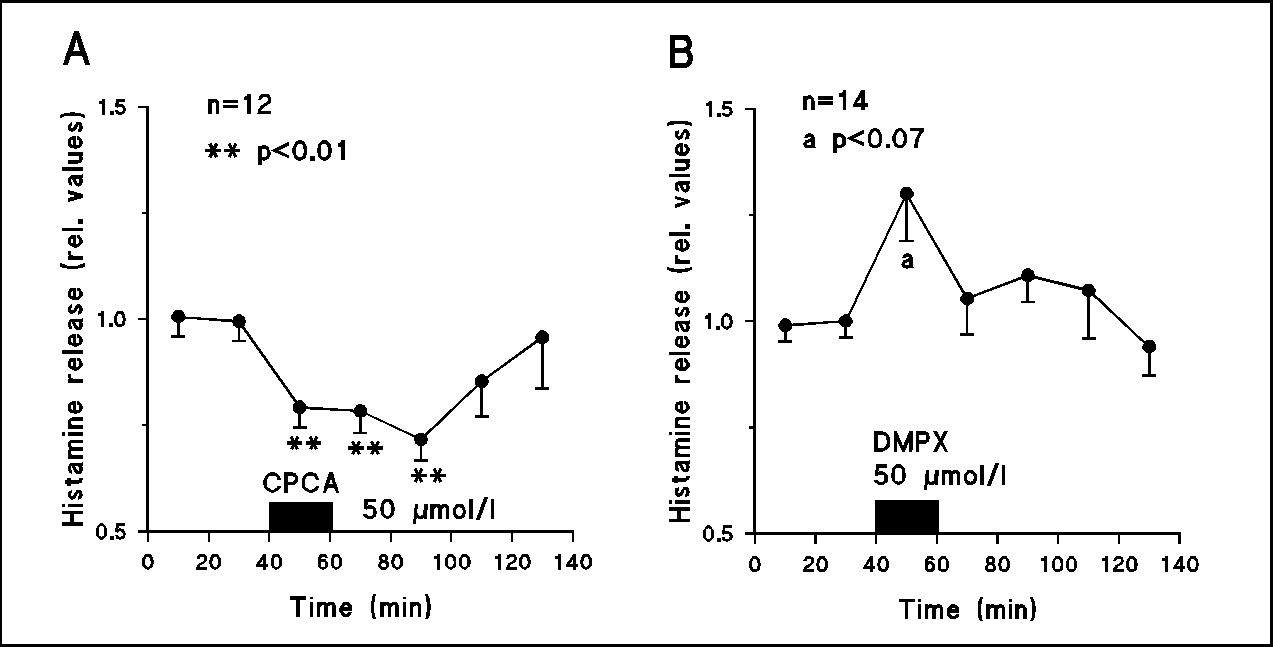
Fig.2
A: Histamine release was also diminished on superfusion with the A2 receptor agonist 5’-(N-cyclopropyl)-carboxamidoadenosine (CPCA, 50 µmol/l). B: The A2 receptor antagonist 3,7-dimethyl-1-propargylxanthine (DMPX, 50 µmol/l) enhanced the release of the amine.
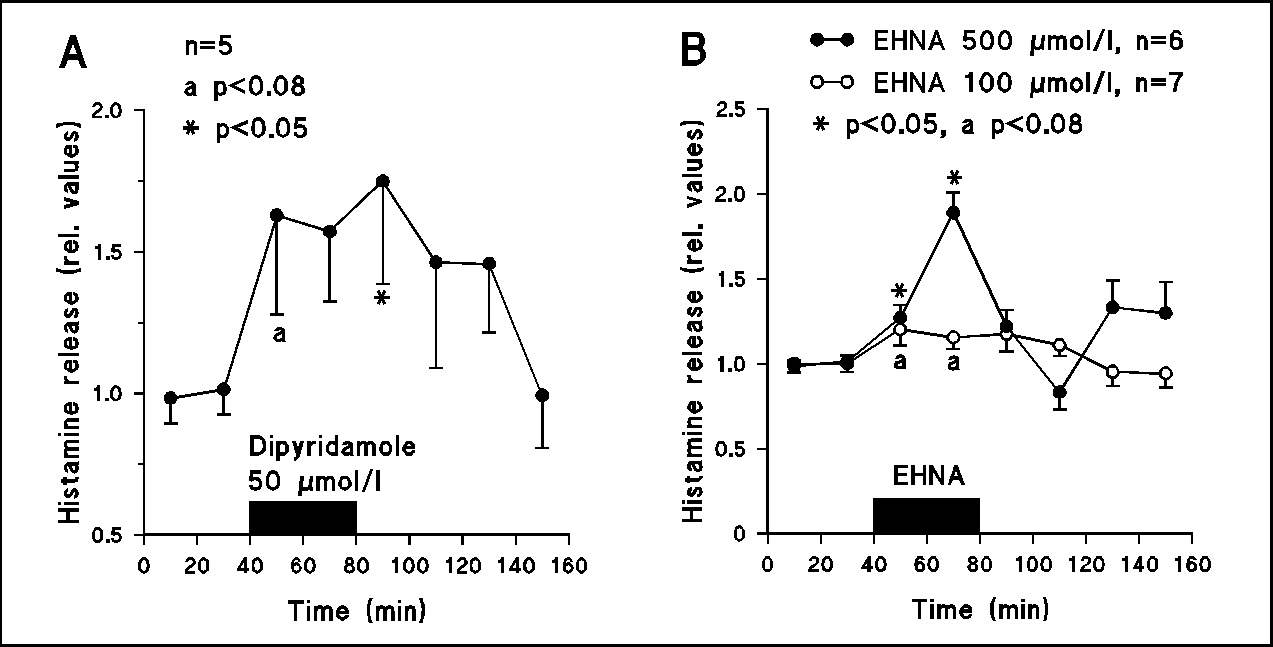
Fig.3
A: Superfusion with dipyridamole (50 µmol/l) which enhances extracellular adenosine concentration by inhibition of adenosine uptake also increased histamine release. B: Superfusion of the anterior hypothalamus with erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA, 100 and 500 µmol/l) which inhibits adenosine deaminase induced a pronounced and concentration-dependent increase in histamine release.
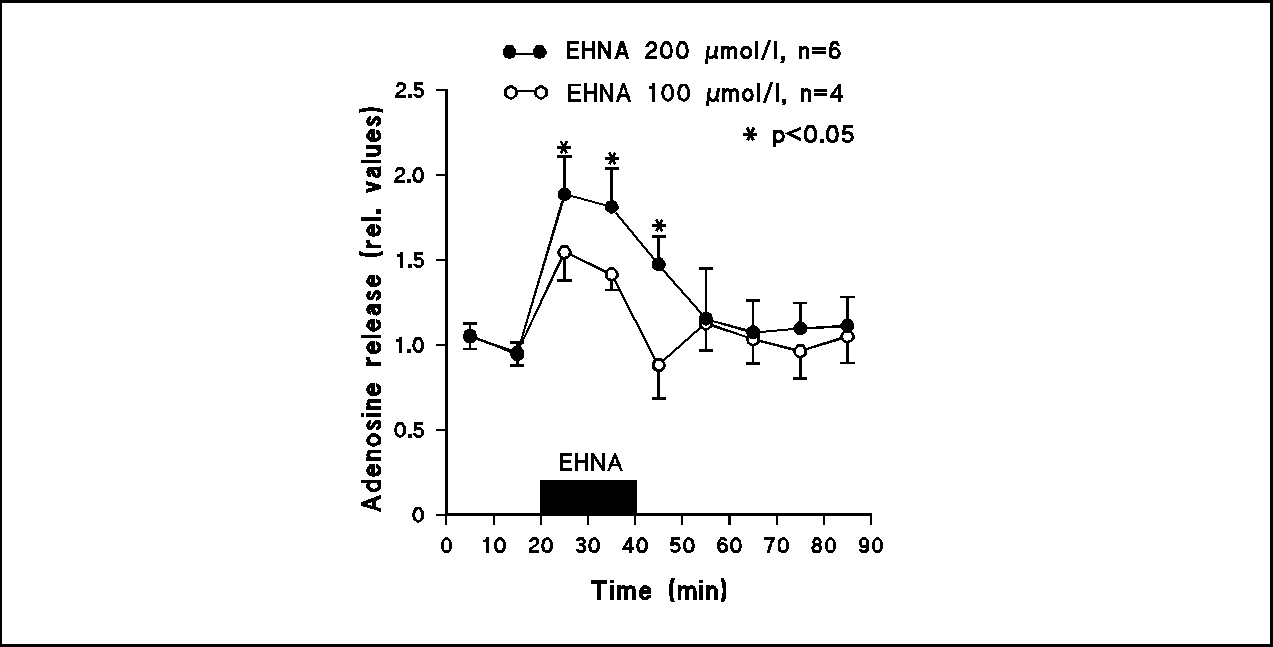
Fig.4
Superfusion with EHNA (100 and 200 µmol/l) enhanced the extracellular adenosine concentration in the hypothalamus.
Discussion and Conclusion
The findings suggest that A1 receptor activation increases, while activation of A2 receptors decreases histamine release. The effects of adenosine receptor antagonists indicate that histamine release is permanently modulated by A1, as well as A2 receptors. The effects of dipyridamole and EHNA on histamine and adenosine release also suggest that adenosine released in the synaptic cleft influences histamine release. Modulation by A1 receptors seems to overrule the opposite modulatory influence of A2 recepors, since elevation of extracellular adenosine level by EHNA or dipyridamole enhances histamine release.
However, it has been reported that adenosine receptors modulate the release of various other neurotransmitters in a way opposite to these findings. A1 receptor stimulation has been found to inhibit the release of several transmitters, whereas A2 receptor activation seems to increase transmitter release (Review: Fredholm and Dunwiddie, 1988). Possibly, adenosine does not influence histaminergic neurons directly, but via modulation of another hypothalamic transmitter which acts in an inhibitory way on histamine release. Candidates for this role are mainly acetylcholine and noradrenaline which are known to inhibit potently brain histamine release (Prast et al. 1991, Prast et al. 1994).
References
- Dunwiddie V, Hoffer BJ (1980) Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br. J. Pharmacol. 69: 59-68
- Dux E., Fastbom J, Ungerstedt U, Rudolphi K, Fredholm BB (1990) Protective effect of adenosine and a novel xanthine derivative propentofylline on the cell damage after bilateral carotid occlusion in the gerbil hippocampus. Brain Res 516: 248-256
- Fastbom J, Fredholm BB (1985) Inhibition of [3H] glutamate release from rat hippocampal slices by L-phenylisopropyladenosine. Acta Physiol Scand 125: 121-123
- Fischer H, Prast H, Philippu A (1995) Adenosine released in the ventral striatum of the rat is modulated by endogenous nitric oxide. Eur J Pharmacol 275: R5-R6
- Fredholm BB, Dunwiddie TV (1988) How does adenosine inhibit transmitter release? Trends Pharmacol Sci 9:130-134
- Hagberg H, Andersson P, La carewicz J, Jacobson I, Butcher I, Sandberg M (1987) Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J. Biol. Chem 49: 227-231
- Lloyd HGE, Spence I, Johnston GAR (1988) Involvement of adenosine in synaptic depression induced by a brief period of hypoxia in isolated spinal cord of neonatal rat. Brain Res 462: 391-395
- Prast H, Fischer H, Fischer H, Prast M, Philippu A (1994) In vivo modulation of histamine release by autoreceptors and muscarinic acetylcholine receptors in the rat anterior hypothalamus. Naunyn-Schmiedeberg’s Arch Pharmacol 350: 599-604
- Prast H, Heistracher M, Philippu A (1991) In vivo modulation of histamine release in the hypothalamus by adrenoceptor agonists and antagonists. Naunyn-Schmiedeberg’s Arch Pharmacol 344: 183-186
- Siggins GR, Schubert P (1981) Adenosine depression of hippocampal neurons in vitro: an intracellular study of dose-dependent actions on synaptic and membrane potentials. Neurosci Lett 23: 55-60
- Winn HR, Rubio R, Curnish RR, Berne RM (1981) Changes in regional cerebral blood flow caused by increases in CSF concentrations of adenosine and 2-chloroadenosine. J Cereb Blood Flow Metab 1: 401-402
| Discussion Board | Previous Page | Your Poster Session |