Neuropharmacology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Protein phosphorylation modulates the expression of specific functions of various proteins and represents a major biochemical mechanism by which cells integrate extracellular signals and maintain homeostasis, cellular functions and survival. The phosphorylation of substrate proteins by protein kinases play a key role in the signal transduction and function of neurons.
Protein kinases are associate with several physiological and pathological states including depression. It has been observed that a chronic treatment of rats with antidepressant reduces cAMP - dependent protein kinase (PKA) activity in soluble fraction whereas increases its activity in particulate fraction from rat brain frontal cortex. It has been also published that antidepressant drugs decrease the activity of protein kinase C (PKC) in soluble and particulate fractions from rat brain cerebral cortex and hippocampus.
The aim of the present study was to investigate the effect of antidepressants and electroconvulsive treatment on the activity of calcium/calmodulin dependent protein kinase II (CaM-KII) in the hippocampus.
Materials and Methods
Subjects:
Wistar rats of initial weight 150 - 200 g were used in our studies. Acclimatized to the colony for one week before the start of treatment. Rats were housed in groups of 5 - 6 per cage with food with and water avaliable ad libitum and kept on a 12 :12 h light/dark cycles. A acute imipramine (IMI 1) and ECS (ECS 1) treatments were given 24 hours before decapitation. In chronically treated group imipramine (IMI X) was injected i.p. at 10 mg/kg once a day for 14 days. Chronic ECS (ECS X) group received 90 mA, 0.5 sec. electric shock every second day for 14 days. Animals were sacrificed 24 h after last treatment.
Tissue preparation:
Following the decapitation brains were fast removed and cooled in ice-cold phosphate bufered saline. Hippocampi were dissected, pooled together and homogenized in glass - glass homogenizer in ice - cold buffer containing protease inhibitors (10 mM Tris - HCl [pH=7.4], 1 mM EDTA, 1mM EGTA,0.5 mM dithiothreitol (DTT), 0.1 mM phenmethylsulfonyl fluoride (PMSF), 10 mg/l leupeptin, 50 mg/l soybean trypsin inhibitor and centrifugated at 120,000 x g for 10 min at 4*C. The supernatant was collected and the pellet was resuspended with the starting volume of homogenization buffer. Protein content was estimated using micro - BCA kit (Piece). Samples were stored in -80 deg C until used.
Assay of CaM-KII:
Activity of CaM-KII was measured using synthetic peptide autocamtide - 3 (AK-3; K-K-A-L-H-R-Q-E-T-V-D-A-L) modelled afer authophosphorylation site of CaM-KII, as specyfic CaM-KII substrate. Phosphorylation reaction was conducted on the phosphocellulose membrane plates Durapore - PH (Millipore [MAHNOB]). The membrane plates were firs prewetted with 100 *l of 1 M Tris - HCl (pH=7.4). 25*l aliquots of particulate ot soluble fraction (containing 3 µg protein) were then incubated with 37.5 µl assay buffer (conaining: 50 mM Tris - HCl [pH=7.4], 0.5 DTT, 20 µM AK-3, 20 *g/ml CaM, 0.2 mM CaCl2, 10 mM MgCl2, 10 *M ATP and 1 &micor;M [* - 32P] ATP [NEN]) in order to determine Ca2+/CaM dependent CaM-KII activity. To determine Ca2+/CaM - independent activity the same reactions in which 1 mM EGTA was substituted for 0.2 mM CaCl2, were performed. All phosphorylation reactions were done in quadruplicates at 30*C for 40 sec., the duration that was demonstrated earlier to provide linear kinetics. Reactions were terminated by washing of the membrane plates four times with an ice - cold 100 mM o - phosphoric acid. Membranes were put in scintilation vials and counting by Cerenkov radiation. CaM-KII was calculated from a differece between Ca2+/CaM dependent and Ca2+/CaM independent activity. For measurment of the effect of antidepressant on CaM-KII activity in vitro the membranes were preinkubated for 10 min with drug ant the CaM-KII activity was measured as described above.
Immunoblot of CaM-KII:
Qantitiation of CaM-KII - semi - quantitive analysis of the * - subunit of CaM-KII. The protein (20 *g) from controland imipramine or ECS treated were separatedon 10% SDS - PAGE followed by electrophoretic transfer on nitrocelulose membrane. 2D5 monoclonal antibody (at 1 : 4000 dilution,[that recognized both the phosphorylated and unphosphorylated * - subunit of CaM-KII]) followed by anti - mouse alkaline phosphatase secondary antibody and NBT and BCIP (Promega), were used to visualise the kinase. Semi - quantitiation of immunostaining was performed by measuring optical density Bio - Rad's GS-670 Imaging Densitometer and Molecular Analyst program.
Analysis of the data:
Data are presented as means * SEM and evaluated by one - way analysis of variance followed by Dunnett multiple comparison test /* « p< 0.01, ** « p<0.001 ,*** « p<0.0001/.
Discussion and Conclusion
Summary:
Repeated (but not acute) imipramine administration significantly decreased CaM-KII activity in soluble fraction from the hippocampus (by 65% reduction), a repeated ECS (the most efficacious antidepressant treatment) produced a similar effect (by 70% reduction).
The decrease of the enzyme activity was accompanied by a proportional decrease (by 60 - 70%) of the amount of a - CaM-KII both after repeated imipramine and ECS treatments.
The single and repeated administration of imipramine induced a significant increase in the activity of CaM-KII in particulate fraction from hippocampus (by 50% and 337% respectively). Single and repeated ECS produced a similar effect (by 22% and 240% increase, respectively).
The amount of a-CaM-KII in particulate fraction was not significantly modified by repeated antidepressant treatment.
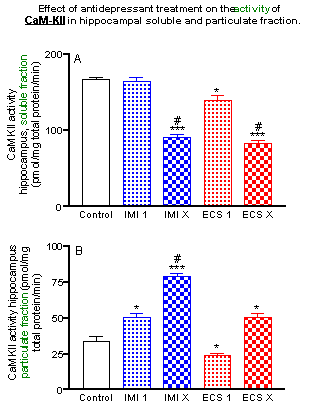
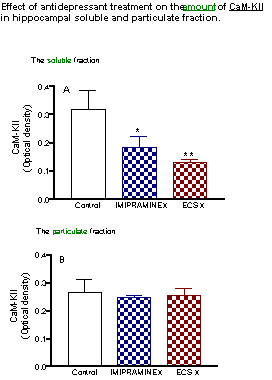
The paralelism between activity and amount of CaM-KII in soluble fraction indicates that specific activity of the enzyme was not changed, suggesting that it is the loss of the enzyme quantity not its activity that account for the decrease of enzymatic activity observed in soluble fraction following chronic antidepressant treatment.
The increase of enzyme activity in particulate fraction coincides with modest increase in the enzyme amount, suggesting that translocated soluble enzyme retains its activity after translocation to particulate fraction and results in superadditive augmentation of particulate enzyme activity.
In vitro phosphorylation assay (data not shown) a mixture of 4mM desipramine (imipramine active metabolite) and 21 mM imipramine (concentrations that were found in the rat brain tissue after 1 hour after last injection in chronic imipramine administration) caused a completly inhibition of CaM-KII activity in particulate fraction. Mixture of imipramine and desipramine at concentrations (±1 mM, [which are found in brain tissue after 24 hours after last injection in chronic imipramine administration]) were without effect on CaM-KII activity.
We concluded, it is possible that imipramine and their metabolite desipramine may influence the CaM-KII activity in a direct post - receptor action.
The locus of CaM-KII translocation and significance and/or mechanism of its activation remain to be tested.
| Discussion Board | Previous Page | Your Poster Session |