Invited Symposium: Cardiac Ischemia Reperfusion
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Protection of ischemic myocardium has long been a focus of research in cardiology. In the very early stages of myocardial ischemia, all jeopardized cells remain viable, but over time cells begin to die. The earlier reflow is established, either by angioplasty or thrombolytic therapy, the greater the degree of salvage. However, in the United States, only about two-thirds of patients with acute myocardial infarction receive thrombolytic therapy. Of those who do, the time to coronary artery reperfusion may average nearly three hours after the onset of chest pain. Therefore, methods to protect ischemic myocardium and to delay cellular necrosis are needed. Studies in many animal models of coronary artery occlusion have suggested that there is a correlation between body, blood and pericardial temperatures with myocardial infarct size (1-3). For example, Chien and coworkers studied the effect of body temperature, in the normothermic range (35-42 degrees C), on infarct size development in rabbits. They found infarct size to be closely correlated with body temperature (1).
Cold cardioplegia has been used by thoracic surgeons for many years to preserve the heart, and we have shown that cold cardioplegia can protect canine myocardium for at least three hours of global ischemia (4). Application of whole-body and global cardiac hypothermia during cardiac surgery are well-established procedures. Hypothermia, often combined with cardioplegia, is thought to protect against anoxia due to its ability to slow cellular metabolism and lower myocardial oxygen demand. The use and effects of hypothermia in the nonworking, globally ischemic heart have been well explored. However, the feasibility of using this concept of cardioprotection in the working heart, subjected to regional ischemia, has not been fully investigated.
Recently we began testing an intervention that consistently reduces myocardial infarct size in an experimental rabbit model to a greater extent than any pharmacologic agent that we have tested. In addition, this intervention is effective even when initiated after the onset of ischemia. Potentially this technique could be developed for use in the clinical realm.
Regional Myocardial Hypothermia (RMH)
The goal of our initial study (5) was to test the hypothesis that a moderate reduction in myocardial temperature (from approximately 39 degrees C to 35 degrees C) would reduce myocardial infarct size. We chose to use a technique that would quickly reduce local myocardial temperature without lowering core temperature. The method we selected was to cool the anterior surface of the heart by applying an ice and water filled bag.
Anesthetized, open-chest rabbits were randomly assigned to one of two groups. In animals receiving RMH (n=14), a bag containing ice and water (0 degrees C) was placed over the exposed heart. The bag remained in place for a 20-minute cooling period, 30 minutes of coronary occlusion and 15 minutes of reperfusion. The bag was then removed and the hearts allowed to rewarm. The total duration of reperfusion was 3 hours. Control rabbits (n=13) received a 20 minute waiting period, 30 minutes of coronary artery occlusion and 3 hours of reperfusion. Both myocardial and core temperatures were measured throughout the protocol. The ischemic risk region of the heart was measured using blue dye, injected at the end of the protocol after reoccluding the artery. Incubating the heart slices in triphenyl-tetrazolium chloride delineated infarct size.
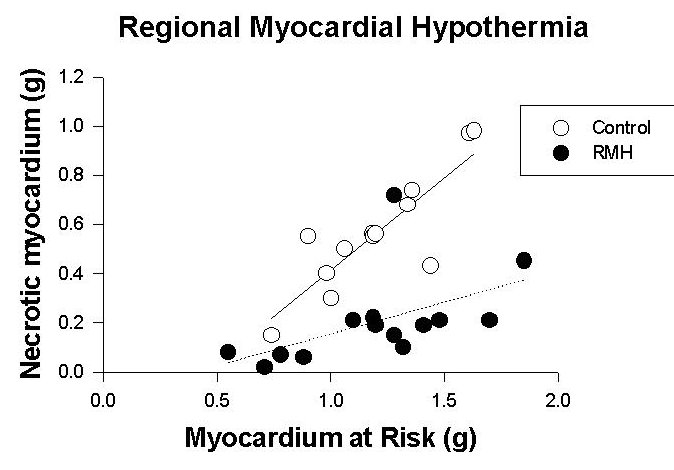
At occlusion, risk zone myocardial temperature in the RMH group was reduced by 2 degrees C from baseline and after 5 minutes of occlusion by 3.6 degrees C. In the control group, myocardial temperature in the risk region remained within 0.3 degrees C of baseline. The ischemic risk region, expressed as a percent of the left ventricle was of similar size in both groups, but while an average of 46 +/- 4% of the risk zone went on to necrosis in control hearts, only 16 +/- 3% of the risk zone became necrotic in hearts cooled before occlusion (p < 0.0001). Thus, hearts in the RMH group exhibited a profound reduction in infarct size compared with control hearts. Risk zone size, expressed in grams, was a strong predictor of the necrotic zone size expressed in grams (p < .003). However, treatment with RMH altered this relationship as can be seen in Figure 1.
 click to enlarge:
:Fig. 1: Scattergram of the necrotic area plotted against the risk area in hearts that were cooled topically starting 30 minutes before coronary artery occlusion (30 min) and normothermic control hearts. Note that on average, for any given size of risk zone, a substantially smaller infarct developed in the cooled hearts.
click to enlarge:
:Fig. 1: Scattergram of the necrotic area plotted against the risk area in hearts that were cooled topically starting 30 minutes before coronary artery occlusion (30 min) and normothermic control hearts. Note that on average, for any given size of risk zone, a substantially smaller infarct developed in the cooled hearts.
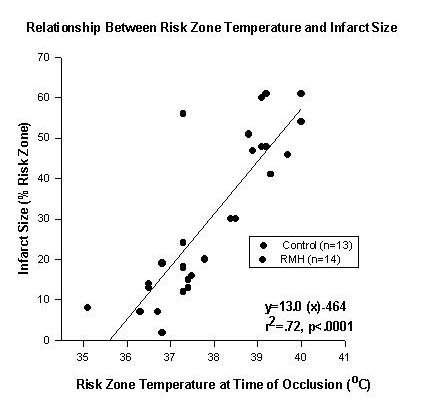
For any given size of ischemic risk region, cooled hearts developed smaller infarcts (p < .0001 by analysis of covariance). Myocardial infarct size expressed as a percentage of the area at risk correlated strongly with myocardial temperature in the risk zone at the time of occlusion (r=.85) as seen in Figure 2.
 click to enlarge:
:Fig. 2: The relationship between myocardial temperature measured just before coronary artery occlusion and eventual infarct size. Infarct size was closely correlated with temperature.
click to enlarge:
:Fig. 2: The relationship between myocardial temperature measured just before coronary artery occlusion and eventual infarct size. Infarct size was closely correlated with temperature.
To determine the effects of various other variables on the development of infarct size, multivariate regression analysis was performed. Tested were the possible predictors of infarct size, including the rate pressure product, myocardial temperature at the time of coronary occlusion, and the size of the risk region. Both myocardial temperature (p < .0003) and size of the risk zone (p < .002) were major predictors of the amount of necrosis that developed. However the rate-pressure product (heart rate times systolic arterial pressure) was not a significant predictor of infarct size development (p=.33). Collateral blood flow is low in this model and is not a predictor of infarct size.
An important observation in this study was that changes in myocardial temperature of only two to four degrees centigrade had profound effects on infarct size progression. The moderate reduction in temperature did not cause vasoconstriction, a potentially confounding effect of hypothermia.
RMH after the onset of ischemia
Is Hypothermia Protective When Initiated After The Onset Of Ischemia?
For use clinically, it is important to determine whether hypothermia is still a protective intervention if used after the onset of ischemia. Thus we designed a study to test the hypothesis that reducing myocardial temperature locally, after coronary occlusion, reduces infarct size (6). Anesthetized rabbits received 30 min of coronary artery occlusion and three hours of reperfusion. Myocardial temperature in the risk zone was monitored. Rabbits were randomly assigned to one of three groups. Group 1 hearts received topical myocardial cooling starting 10 min after coronary occlusion (n=11), group 2 hearts received cooling starting 25 min after coronary occlusion (n=11), and group 3 hearts, control, received no intervention (n=10).
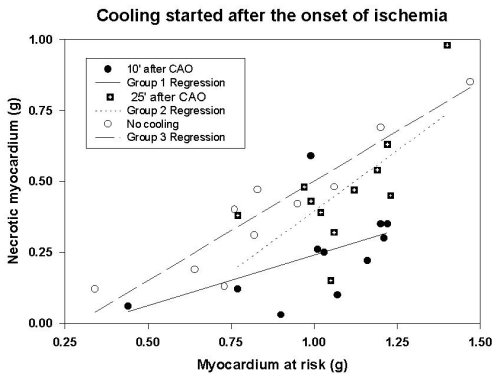
Risk zone temperature was similar in all groups at the time of occlusion. The cooling maneuver produced a rapid reduction in temperature in the risk region. In group 1, myocardial temperature was reduced an average of 6.3 degrees C between 10 and 15 min of coronary artery occlusion; myocardial temperature in group 2 was reduced an average of 5.9 degrees C between 25 and 30 min of coronary artery occlusion. Cooling was maintained until 15 min of reperfusion. Myocardial temperature in group 3 remained within 0.3 degrees C of baseline during coronary artery occlusion and into reperfusion. Mean core temperature was similar in all groups. Although the ischemic risk region was of similar size in all groups, cooling started 10 min after occlusion (group 1) resulted in a significant reduction in infarct size, expressed as a percent of the risk region, compared with the control group (23 +/- 4% vs. 44 +/- 4% of the risk region); however cooling just before reperfusion (group 2) failed to modify infarct size compared with the controls (43 +/- 4% and 44 +/- 4% of the risk region, respectively), as seen in Figure 3
 click to enlarge:
:Fig. 3: Scattergram of the necrotic area plotted against the risk area in hearts cooled after the onset of ischemia. Group 1 hearts-cooling initiated 10 min after coronary occlusion; Group 2 hearts- cooling initiated 25 min after coronary artery occlusion (5 min before reperfusion); Group 3 hearts- no cooling (control).
click to enlarge:
:Fig. 3: Scattergram of the necrotic area plotted against the risk area in hearts cooled after the onset of ischemia. Group 1 hearts-cooling initiated 10 min after coronary occlusion; Group 2 hearts- cooling initiated 25 min after coronary artery occlusion (5 min before reperfusion); Group 3 hearts- no cooling (control).
These results show that reducing myocardial temperature reduces infarct size even when the reduction in temperature is initiated after the onset of ischemia. However, it is important that the reduction in temperature be produced as early as possible following coronary artery occlusion. It appears that the mechanism for the beneficial effects of hypothermia is not a reduction in reperfusion injury.
Is Hypothermia A Useful Therapy In Long-Duration Myocardial Ischemia ?
Some cardiac interventions reduce myocardial infarct size when the duration of ischemia is short but lose their effectiveness when the ischemic period is extended. Is RMH protective with prolonged ischemia? To test this, rabbits received two hours of coronary occlusion followed by three hours of reperfusion (7). In the RMH group (n=14), RMH was initiated 30 minutes into the coronary artery occlusion. Control hearts (n=12) received no intervention.
Myocardial temperatures were similar in both groups at baseline and at 30 minutes of occlusion, before the start of RMH. During the remaining period of ischemia (90 minutes) myocardial temperature was reduced to 30 +/- 5 degrees C in RMH hearts compared with 39 degrees C in controls. In control hearts, infarct size after two hours of ischemia had extended to comprise 72 +/- 3% of the risk zone, but in RMH hearts infarct size was only 59 +/- 3% of the risk zone (p < .004).
The longer duration of ischemia resulted in an extension in the amount of necrosis, such that after two hours of ischemia, infarct size in controls comprised about three-quarters of the risk area. However, even when RMH was begun 30 minutes after the start of ischemia, it still significantly reduced infarct size by 18%.
Summary and Conclusions
Data from our studies support the important role of myocardial temperature level in the progression of necrosis and suggest the potential use of hypothermia as a therapeutic maneuver to protect regionally ischemic myocardium. In humans, the development of infarction occurs more slowly, as humans with coronary artery disease generally have more collateral blood flow than animal models such as the rabbit and rat, which have very little. It is known that the rate of development of infarction is directly correlated with the level of collateral flow. It is probable that in humans the application of hypothermia would be beneficial even if initiated later in the ischemic process. However, better techniques to lower cardiac temperature must be developed.
The mechanism for the protective effect of regional hypothermia in the ischemic, beating heart remains to be determined. Although our data shows that the beneficial effects are independent of changes in heart rate or blood pressure, a possible protective effect of hypothermia is a reduction in high energy phosphate utilization in the myocardium. This may occur in the risk region itself, or be due to reduced contractility (and metabolic demand) in the border zone. It has been shown that lowering the temperature from 40-28 degrees C slows the rate of high energy phosphate utilization in dog hearts (8 ). In the liver of rabbits, topical hypothermia of 30 degrees C has been shown to significantly protect ATP levels at 15 and 30 minutes of ischemia (9).
There is considerable data to suggest that lowering of temperature is beneficial in the setting of myocardial ischemia. However, before this intervention can be used clinically, improved techniques must be developed to reduce myocardial temperature. In larger animals it takes several hours to lower core temperature by cooling the entire body topically (10). The technique of topical cooling also has limited application, as the heart must be exposed. More promising are techniques such as hypothermic pericardioperfusion (11), which could potentially be performed using a catheter inserted subxyphoid, for example, before minimally invasive cardiac surgery. By using a relatively atraumatic pericardial catheter introducer, intrapericardial perfusion of cold fluid could even be considered in patients with acute myocardial infarction. Also the method used by Miki et. al. to lower body temperature by using a heat exchanger inserted between a peripheral artery and vein holds potential (12).
In conclusion, RMH resulted in a significant infarct size reduction compared with normothermic control hearts. This reduction occurred when RMH was initiated either before or after the onset of ischemia. RMH protected myocardium even when the duration of ischemia was prolonged to 2 hours, however RMH was not beneficial when begun just before reperfusion. The reduction in infarct size was not a result of differences in body temperature, ischemic risk area size, hemodynamics, nor regional myocardial blood flow, which were comparable among control and treated groups in all studies. Started before or after ischemia, RMH is an effective technique to protect the heart, reducing necrosis resulting from ischemia.
References
1. Chien G L, Wolff R A, Davis RF, Van Winkle DM. (1994)"Normothermic range" temperature affects myocardial infarct size. Cardiovasc Res 28:1014-1017
2. Duncker, DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. (1996) Effect of temperature on myocardial infarction in swine. Am J Physiol 270:H1189-H1199
3. Schwartz LM, Verbinski SG, Vander Heide RS, Reimer KA. (1997) Epicardial temperature is a major predictor of infarct size in dogs. J Mol Cell Cardiol 29:1577-1583
4. Przyklenk K., Aoki A, Bellows S, Klinedinst D, Zubinate P, Jr, Hale SL, Simkhovich BZ, Kloner RA, and Kay GL. (1994) Stunned myocardium following prolonged cardiopulmonary bypass: Effect of warm versus cold cardioplegia in the canine model. J Card Surg 9 [Suppl]:506-516
5. Hale SL, Kloner RA. (1997)Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am J Physiol 273:H220-H227
6. Hale SL, Dave RH, Kloner RA. (1997) Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol 92:351-357
7. Hale SL, Kloner RA. (1998) Myocardial temperature reduction attenuates necrosis after prolonged ischemia in rabbits. Cardiovasc Res (in press)
8. Carrier M, Tourigny A, Thorbe N, Montpetit M, Khalil A, Solymoss BC, Pelletier LC. (1994) Effects of cold and warm blood cardioplegia assessed by myocardial pH and release of metabolic markers. Ann Thorac Surg 58:764-67
9. Eidelman Y, Glat PM, Pachter HL, Cabrera R, Rosenberg C. (1994) The effects of topical hypothermia and steroids on ATP levels in an in vivo liver ischemia model. J Trauma 37:677-681
10. Abendschein DR, Tacker WA, and Babbs CF. (1978) Protection of ischemic myocardium by whole-body hypothermia after coronary artery occlusion in dogs. Am Heart J 96:772-780
11. Dave RH, Hale SL, Kloner RA. (1998) Hypothermic, closed circuit pericardioperfusion: a potential cardioprotective technique in acute regional ischemia. JACC 1667-1671
12.Miki T, Cohen MV, Downey JM. (1997) Hypothermia's cardioprotective effect is additive to that of ischemic preconditioning. Circulation 96 (Suppl):I-687
| Discussion Board | Previous Page | Your Symposium |