Invited Symposium: Quinones and Other Reactive Oxygen Species in Neurobiologic, Apoptotic, and Neurotoxic Processes
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Neurodegeneration is a process which occurs during normal ageing, in connection with several neurodegenerative diseases and in the presence of various chemical agents. Although it is generally accepted that free radicals are involved in the neurodegenerative process occurring in the dopaminergic neurons nigro-striatal system in Parkinson's disease, the exact mechanism of neurodegeneration in vivo is still unknown. One possible source of free radicals may involve the oxidation of dopamine to the corresponding o-quinone (aminochrome) (1) and its activation to reactive oxyygen species (2,3). Dopamine, like catecholamines, can be oxidized in the presence of either oxygen at high pH, or other oxidants, to the corresponding ortho-quinones (4-6).
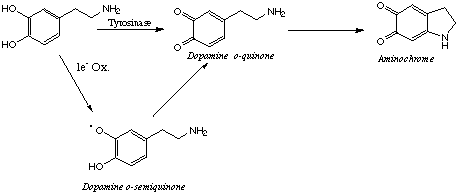
This oxidative process forms the precursor for the melanin pigment. However, under pathological conditions dopamine metabolism can be diverted towards aberrant oxidative pathway. Dopamine and L-dopa have been reported to induce apoptosis (7-9) but the underlying molecular mechanism is unclear. In addition, both dopamine and L-dopa have been reported to be toxic to neurons. The recent renewal of interest in free radicals-related neurotoxicity of dopamine and its oxidative products, o-quinones, comes from the evidence for a potential link between oxidative stress and apoptosis ( 10-15).
One- and two-electron reduction of aminochrome catalyzed by NADPH cytochrome P450 reductase and DT-diaphorase have been studied, respectively. (14, 5). It is of interest to note that both NADPH-cytochrome P-450 reductase and DT-diaphorase have been reported to occur in dopaminergic neurons (10, 11).
One-electron reduction of aminochrome
NADPH-cytochrome P450 reductase catalyzes one-electron reduction of aminochrome to o-semiquinone which seems to be extremely reactive. The continuos NADPH oxidation and oxygen consumption reveal its autoxidation (2). Aminochrome o-semiquinone autoxidize by reducing oxygen to superoxide radicals giving rise a redox cycling:
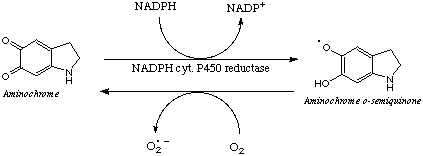
This redox cycling will continue until NADPH or oxygen is depleted. It is of interest to note that very low concentrations of aminochrome can produce a large amount of reactive oxygen species due its ability to redox cycling. We have to notice that depletion of NADPH will result in the accumulation of oxidized glutathione (GSSG) and depletion of reduced glutathione (GSH) which is an important cellular antioxidant in the cell. In addition, depletion of NADH will inhibit the synthesis of ATP in the mitochondrial transport chain.
The high reactivity of aminochrome o-semiquinone was strong supported by ESR studies where the o-semiquinones were stabilized by Zn2+(3). Dopamine oxidized by tyrosinase was used to perform ESR studies to detect o-semiquinone radical signal during one-electron reduction. A mixture of two spectra containing aminochrome o-semiquinone and dopamine o-semiquinone was obtained since tyrosinase catalyzes the formation of both dopamine o-quinone and aminochrome. NMR studies showed that 50 % of reaction mixture during dopamine oxidation catalyzed by tyrosinase was dopamine o-quinone after 4 hours. This result suggest that the cyclization process of the amino-chain of dopamine o-quinone is not rapid and spontaneous process as during dopamine oxidation catalyzed by Mn3+-pyrophosphate. Of these mixture of ESR spectra only dopamine o-semiquinone spectra was stabilized and accumulated during 10 min. The stabilized ESR signal after 10 min was exactly the same as the ESR signal obtained during one-electron oxidation of dopamine to dopamine o-semiquinone catalyzed by lactoperoxidase in the presence of hydrogen peroxide (3). Aminochrome o-semiquinone ESR signal was found to be very unstable because only traces of this signal was detected after a min of incubation. These results are a good evidence that NADPH cytochrome P450 reductase also catalyzes the one-electron reduction of dopamine o-quinone. The instability of aminochrome o-semiquinone contrast with the stability of dopamine o-semiquinone suggesting that aminochrome o-semiquinone is the specie responsible for the formation reactive oxygen species during the oxidative process of dopamine. The stability of dopamine o-semiquinone in the presence of oxygen suggest that the disproportionation reaction of two dopamine o-semiquinone to form dopamine o-quinone and dopamine o-semiquinone is more relevant than reaction with oxygen.
Another important feature of aminochrome o-semiquinone is that the removal of superoxide radicals and hydrogen peroxide present in the incubation mixture result in the increase of the autoxidation rate (Fig.1). This increase in the autoxidation rate of aminochrome o-semiquinone can be explained by the possible competition between dioxygen (O2) and superoxide radicals and hydrogen peroxide to react with the o-semiquinone. Therefore, the presence of SOD and catalase which remove both superoxide radicals and hydrogen peroxide will result in the increase of the autoxidation rate of aminochrome o-semiquinone. These results suggest a clear prooxidant role of these antioxidant enzymes during one-electron reduction of aminochrome.
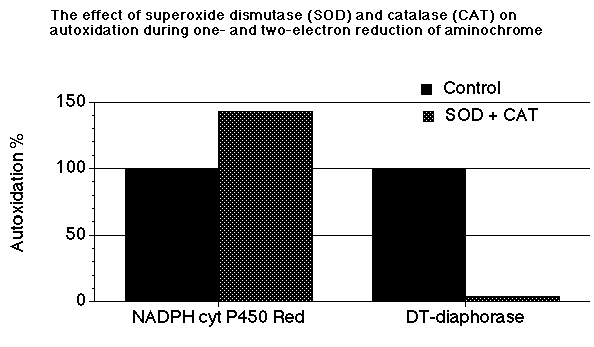 Fig. 1. The effect of superoxide dismutase and catalase on the autoxidation of aminochrome during one- and two-electron reduction of aminochrome catalyzed by NADPH cytochrome P450 reductase and DT-diaphorase, respectively (BRC).
Fig. 1. The effect of superoxide dismutase and catalase on the autoxidation of aminochrome during one- and two-electron reduction of aminochrome catalyzed by NADPH cytochrome P450 reductase and DT-diaphorase, respectively (BRC).
Two-electron reduction of aminochrome
DT-diaphorase catalyzes two-electron reduction of aminochrome to o-hydroquinone which also autoxidize in the presence of oxygen resulting in the formation superoxide radicals and hydrogen peroxide. However, the autoxidation rate is very low compared to aminochrome o-semiquinone autoxidation rate.
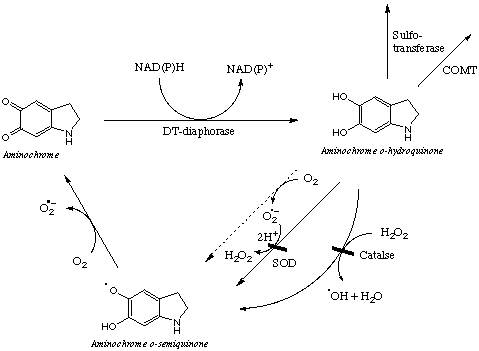
The chemistry of autoxidation of o-hydroquinone was found to be completely difference since superoxide radicals and hydrogen peroxide are mainly responsible of autoxidation of the o-hydroquinone (96% of the total oxygen-dependent autoxidation) (3, 14). Therefore, the addition of superoxide dismutase and catalase to the incubation mixture inhibited the formation of reactive oxygen species during the reduction of aminochrome catalyzed by DT-diaphorase by preventing o-hydroquinone autoxidation (Fig. 1). This antioxidant role of superoxide dismutase and catalase on autoxidation of o-hydroquinone during reduction of aminochrome catalyzed by DT-diaphorase contrast with their prooxidant role on aminochrome o-semiquinone autoxidation during aminochrome reduction catalyzed by NADPH-cytochrome P450 reductase. These results support the proposed protective role of DT-diaphorase in the presence of SOD and catalase against aminochrome-induced oxygen toxicity in the neurodegenerative process of dopaminergic neuron systems (5,14). It is of interest to note the importance to reduce aminochrome to o-hydroquinone which can be conjugated by sulfotransferase to an unreactive species, which can be excreted from the cell.
Conclusions
1) The results presented above strong suggest that the reduction of aminochrome to o-semiquinone is the step where the reactive oxygen species, which may be responsible for the degenerative process of dopaminergic system, are formed. Aminochrome o-semiquinone is extremely reactive and autoxidize in the presence of oxygen giving rise a redox cycling process which is accelerated by the antioxidant superoxide dismutase and catalase (2).
2) The other reaction where reactive oxygen species can be formed is during one-electron oxidation of dopamine or one-electron reduction of dopamine o-quinone. However, enormous difference in the stability of dopamine o-semiquinone compared to aminochrome o-semiquinone in the presence of oxygen strong support the idea that dopamine o-semiquinone disproportionate to dopamine and dopamine o-quinone instead to react with oxygen and produce reactive oxygen species.
3) DT-diaphorase is an antioxidant enzyme which may protect the cell against aminochrome toxicity by competing with one-electron quinone reductases to reduce aminochrome to o-hydroquinone which can be conjugated by sulfotransferase and COMT. In addition, superoxide dismutase and catalase prevent the possible autoxidation of o-hydroquinone (2).
References
- 1. Graham, D.G., Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones, Mol. Pharmacol. 14, 633-643 (1978).
- 2. Baez, S., Linderson, Y. and Segura-Aguilar, J., Superoxide dismutase and catalase enhance autoxidation during one-electron reduction of aminochrome by NADPH-cytochrome P-450 reductase, Biochem. Mol . Med. 54, 12 18 (1995)
- 3. Segura-Aguilar, J, Metodiewa, D. and Welch, C.J. Metabolic activation of dopamine oquinones to o-semiquinones by NADPH cytochrome P450 reductase may play an important role in oxidative stress and apoptotic effects. Biochim. Biophys. Acta 1381, 1-6 (1998)
- 4. Hawley, M.D., Tatawawadi, S.V., Piekarski, S. and Adams, R.N., Electrochemical studies of the oxidation pathways of catecholamines, J. Am. Chem. Soc. 89, 447-450,(1967).
- 5. Harrison, W.H., Whisler, W.W., and Hill, B.J., Catecholamine oxidation and ionization properties indicated from the H- release, tritium exchange, and spectral changes which occur during ferricyanide oxidation, Biochemistry 7, 3089-3093, (1968).
- 6. Segura-Aguilar, J. and Lind, C., On the mechanism of the Mn3+-induced neurotoxicity of dopamine prevention of quinone-derived oxygen toxicity by DT-diaphorase and superoxide dismutase, Chem.-Biol. Interactions 72, 309-324, (1989) .
- 7. Offen, D., Ziv, I., Gorodin, S., Barzilai, A., Malik, Z. and Melamed, E. Dopamine-induced programmed cell death in mouse thymocytes. Biochim. Biophys. Acta 1268, 171-177, (1995)
- 8. Walkinshaw, G.and Waters, C.M. Induction of apoptosis in catecholaminergic PC12 cells by L-DOPA. Implications for the treatment of Parkinson's disease. J. Clin. Invest. 95, 2458- 2464 (1995)
- 9. Ziv, I., Melamed, E., Nardi, N., Luria, D., Achiron, A., Offen, D. and Barzilai, A. Dopamine induces apoptosis-like cell death in cultured chick sympathetic neurons--a possible novel pathogenetic mechanism in Parkinson's disease Neurosci. Lett. 170, 136-140. (1994)
- 10. Lai, C.T., Yu, P.H., Dopamine and L-dopa induced cytotoxicity towards catecholaminergic neuroblastoma SH-5Y5Y cells: effects of oxidative stress and antioxidative factors, Biochem. Pharmacol. 53, 363-372 (1997)
- 11. Simantov, R., Binder, H., Tratovitsky, T., Tauber, M., Gasbbay, S., Porat, S., Dopamine-induced apoptosis in human neuronal cells: inhibition by nucleic acids antisense to the DA transporter, Neuroscience 74, 39-50 (1996);
- 12. Offen, D., Ziv, J., Barzilai, A., Gorodin, S., Glater, E., Hoochman, A., Melamed, E., Dopamine-melanine induces apoptosis in PC 12 cells: possible implications for the etiology of Parkinsoonīs disease. Neurochem. Int. 31, 207-216 (1997);
- 13. Velez-Pardo, C., Del Rio, M.J., Verschueren, H., Ebinger, G., Vauguelin, G. Doopamine and iron induce apoptosis in PC 12 cells. Pharmacol. Toxicol. 80, 76-84 (1997).
- 14. Ben-Schacher, D., Zuk, B., Glinka, Y. Dopamine neurotoxicity: inhibition of mitochondrial respiration. J. Neurochem. 64, 718-723 (1995) 15. Jenner, P., Olanow, C.W.. Oxidative stress and pathogenesis of Parkinsonīs disease. Neurology 17, 161-170 (1996)
- 16. Segura-Aguilar, J., Baez, S., Widersten, M., Welch C.J., and Mannervik, B., Human Class glutathione Transferases, in particular isoenzymes M2-2, catalyze detoxication of dopamine metabolite aminochrome. J. Biol. Chem 272, 5727-5731 (1997)
- 17. Baez, S., Segura-Aguilar, J., Widersten, M., Johansson, A-S., and Mannervik, B., Glutathione transferases catalyze the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem . J 342, 25-28 (1997)
| Discussion Board | Previous Page | Your Symposium |