Invited Symposium: Intracellular Traffic of Organelles
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Cystic fibrosis is one of the most prevalent lethal genetic diseases in the Caucasian population. The CF gene product, the cystic fibrosis transmembrane conductance regulator (CFTR), is a polytopic integral membrane protein which belongs to the ATP binding cassette (ABC) transporter family and functions as a cAMP-dependent protein kinase (PKA) activated chloride channel at the apical surface of epithelium.
Immunolocalization and functional studies indicate that CFTR resides in the endosomal compartment. Cell surface biotinylation experiments indicated that CFTR undergoes an efficient internalization in both polarized and non-polarized cells (1, 2). PKA-mediated phosphorylation was reported to evoke the translocation of CFTR from the endosomal compartment to the plasma membrane in T84 epithelia expressing CFTR constitutively (3), and in polarized and non-polarized cells expressing an epitope-tagged version of CFTR (4-7). Finally, quantitative immunocytochemistry showed that CFTR is translocated upon acute hormone stimulation of primary shark rectal gland epithelia (8).
To delineate the mechanism of CFTR endocytosis and the sorting signal(s) responsible for the endocytosis, we characterized the internalization pathway and the endosomal sorting signals at the cytoplasmic tails using intact CFTR and chimeric (Tac-CFTR) molecules. Functional association of CFTR with newly formed endosomes was determined by a rapid endosomal pH dissipation (9) in CHO-BQ1 cells stably expressing CFTR. The role of clathrin-dependent pathway in CFTR endocytosis was evaluated in cells exposed to hypertonic medium, potassium depletion or intracellular acid load. CFTR internalization was also monitored by cell-surface biotinylation of metabolically labeled cells. The role of N- and C-terminal cytoplasmic tails in CFTR endocytosis was examined by immunomicroscopy and 125I-labeled antibody uptake assay using a chimeric approach replacing the cytoplasmic tail of interleukin-2 receptor a-chain (Tac), a plasma membrane resident protein, with the N- or the C-terminal tail of CFTR.
Inhibition of clathrin-coated vesicle formation prevented CFTR endocytosis by 50-90%. Following the inhibition, 50-90% of CFTR molecules were excluded from endosomes. Not only the rate of internalization but also the size of internal CFTR pool accessible to biotin was diminished upon stimulation of protein kinase A (PKA) and protein kinase C (PKC). Consistently, both the N- and the C-terminal tails of CFTR containing putative Tyr-based internalization signals confer highly efficient internalization (10%/min-1 of cell surface chimeras) to the plasma membrane resident Tac-reporter protein. Internalization of the Tac-CFTR chimeras was also abrogated by inhibition of clathrin-coated vesicle formation.
These results suggest that internalization of CFTR occurs by clathrin-coated vesicles, which may be regulated by protein phosphorylation. The cytoplasmic tails of CFTR presumably play an important role in this process.
Materials and Methods
Biotinylation of CFTR
Cells overexpressing CFTR were radioactive labeled with [35S]-methionine and [35S]-cysteine and chased. Cell surface proteins were biotinylated with NHS-SS-biotin on ice or at 37°C.
Construction of TacT-CFTR chimeras
Cytoplasmic tail of Tac was truncated, and replaced with PCR fragment of N- or C-terminal tail of CFTR. Alanine substitution mutagenesis was done by site directed mutagenesis using single or double stranded DNA as template, or PCR. Constructs were verified by DNA sequencing.
Inhibition of clathrin-dependent endocytosis
Clathrin-coated vesicle formation was inhibited by incubating cells in hypertonic medium or by depletion of cytosolic potassium. Cell viability after potassium depletion was tested with Trypan blue exclusion. Reversibility of the hypertonic exposure was verified by H+-efflux rate measurement after reincubating the cells in iso-osmotic medium.
Internalization assays
Biotinylated surface proteins were internalized at 37°C for designated periods. Surface-remaining biotin was stripped with 2-mercaptoethanesulphonic acid (MESNA). Biotinylated CFTR was immunoisolated with anti-CFTR antibodies, and subsequently with streptavidin-Sepharose, and visualized with fluorography.
Internalization of the TacT-chimeras were monitored directly by the uptake of a fluorophore-conjugated anti-Tac antibody. Quantitative assessment was done by measuring the amount of anti-Tac antibody on the cell surface before and after internalization with an iodinated secondary antibody.
Results
Results and Discussion
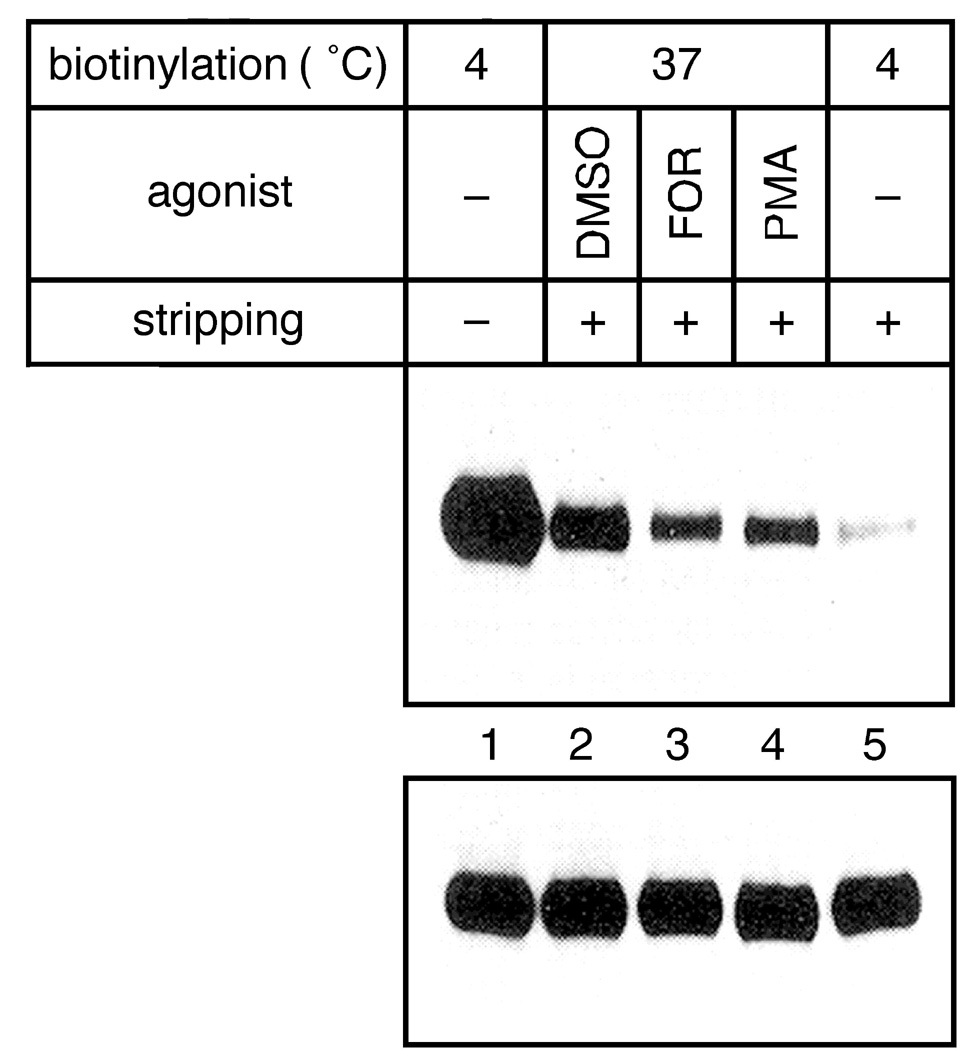
To investigate internalization of CFTR, radioactive labeled CHO-BQ2 cells overexpressing CFTR were biotinylated on ice or at 37°C. Internalization was done at 37°C for 0-30 min. When PKA activity was stimulated with forskolin during biotinylation, the intracellular CFTR pool labeled for 30 min was diminished by 40% compared to the untreated control (Fig. 1). The PKC activation with phorbol 12-myristate 13-acetate (PMA) had similar effect. The results suggest that phosphorylation induced by PKA and PKC facilitate CFTR translocation from an intracellular compartment to cell surface by inhibiting its internalization and stimulating its exocytosis. About 15% of cell surface-labeled CFTR were endocytosed during the first three minutes indicating a fast internalization (data not shown). Lower half of the fluorograph in Fig. 1 represents 10% of the total CFTR immunoprecipitate, to verify the reproducibility of CFTR labeling in different samples. The internalization process of CFTR is dependent upon the formation of clathrin coated vesicles. Inhibition of clathrin-dependent endocytic pathway by three means nearly completely abrogated the retrieval of CFTR into the newly formed endosomes (data not shown).
 click to enlarge
Fig. 1: PKA and PKC promote translocation of biotinylated CFTR.
click to enlarge
Fig. 1: PKA and PKC promote translocation of biotinylated CFTR.
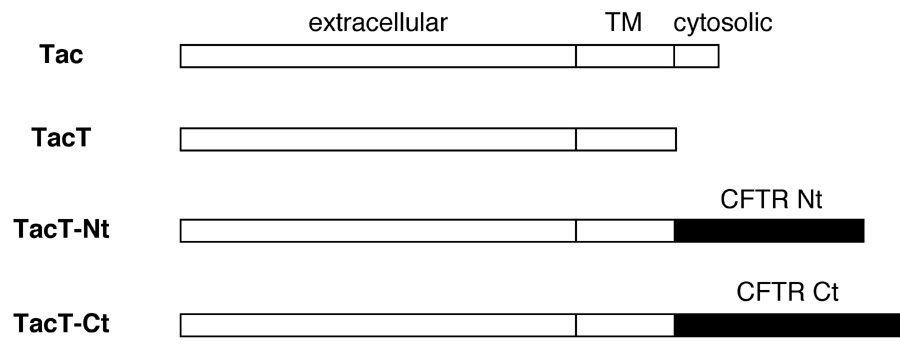
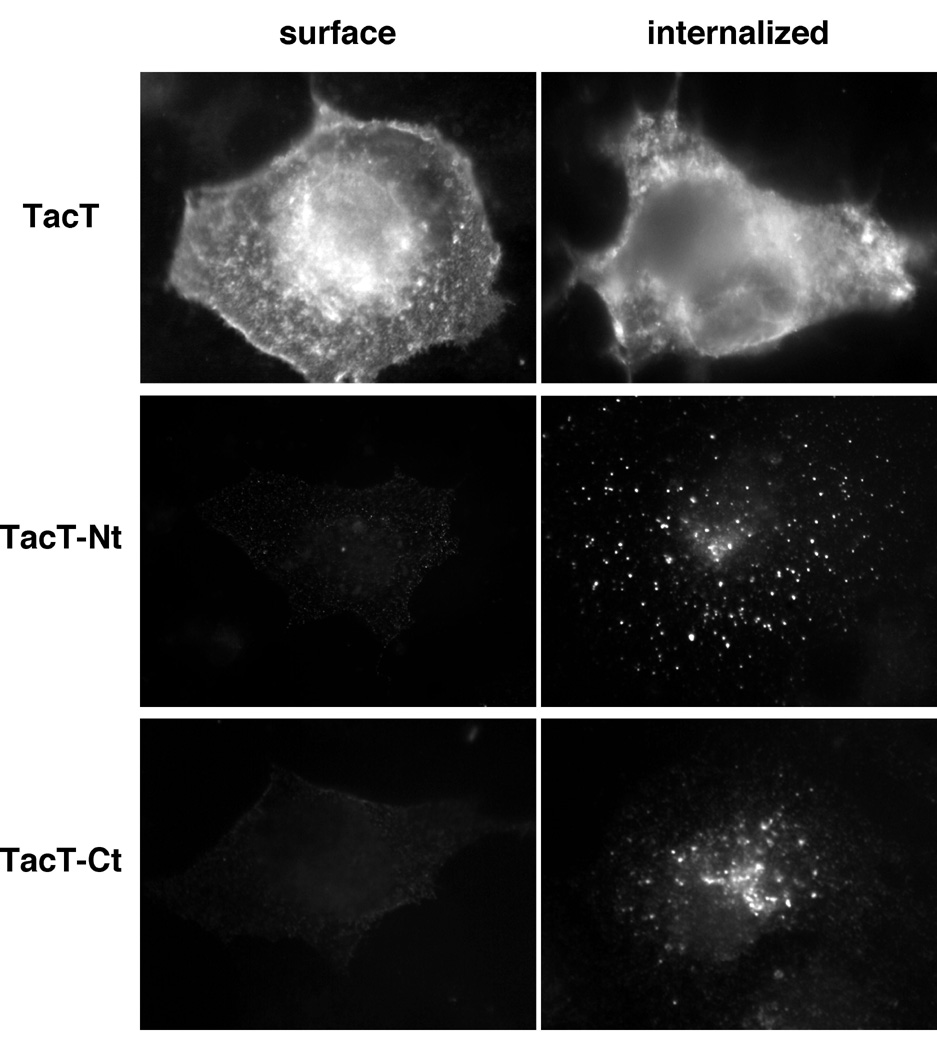
To test whether the cytoplasmic tails play a role in the endocytosis of CFTR, N- or C-terminal tail of CFTR was fused to the C-terminal cytoplasmic tail-truncated Tac (TacT) to form Tac-CFTR chimeras (TacT-Nt and TacT-Ct respectively) (Fig. 2A). TacT and the chimeras transiently expressed in COS-7 cells were allowed to internalize with an FITC-conjugated anti-Tac antibody for 40 min at 37°C. TacT predominantly remained at the plasma membrane. In contrast, the chimeras were very efficiently internalized, indicating that both N- and C-terminal cytoplasmic tails of CFTR contain internalization signal(s) (Fig. 2B).
 click to enlarge
Fig. 2A: Tac-CFTR chimera constructs.
click to enlarge
Fig. 2A: Tac-CFTR chimera constructs.
 click to enlarge
Fig. 2B: Cytoplasmic tails of CFTR confer endocytic signals to TacT.
click to enlarge
Fig. 2B: Cytoplasmic tails of CFTR confer endocytic signals to TacT.
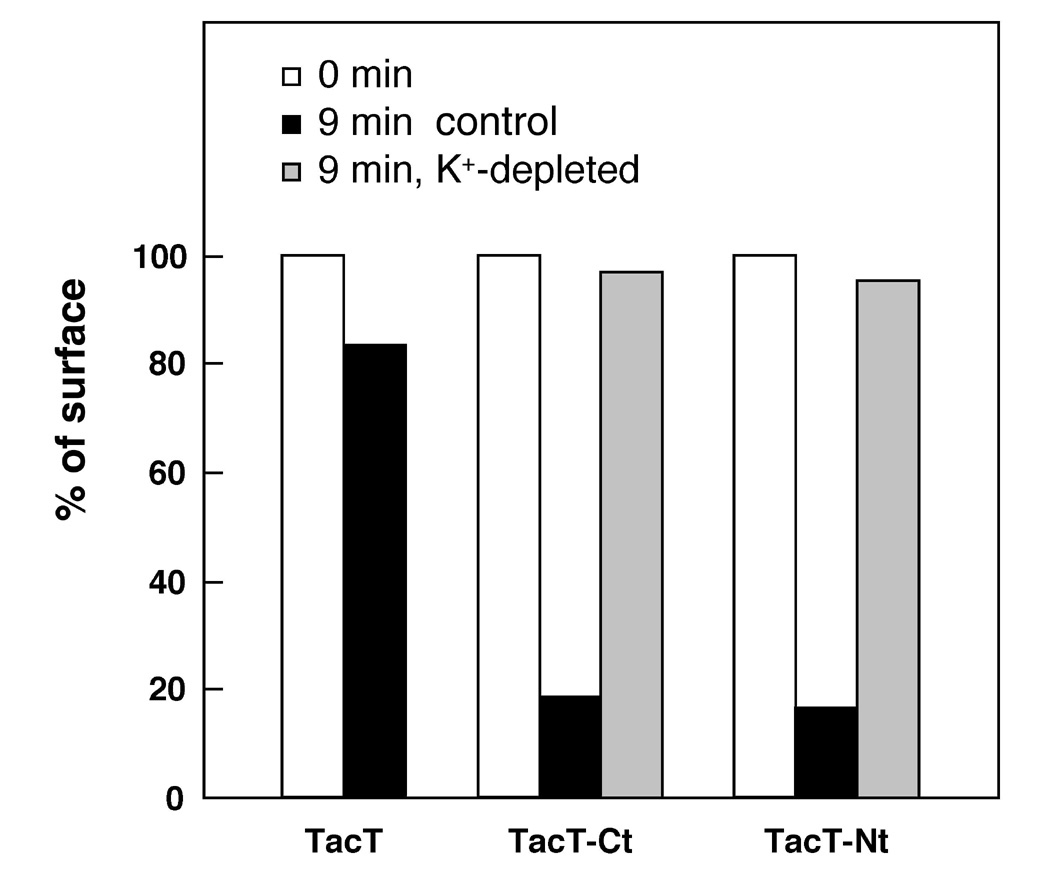
According to quantitative internalization assay of the Tac-chimeras using surface anti-Tac antibody uptake measured by 125I-labeled secondary antibody, both TacT-Nt and TacT-Ct were internalized with high efficiency (~10% min-1 of cell surface Tac-CFTR chimeras), comparable to that of transferrin receptor (Fig. 3). This internalization occurs also via the clathrin-dependent endocytic pathway, consistent with the above observation. Internalization of the chimeras was completely blocked upon arresting of clathrin coated vesicle formation by cytosol potassium depletion (Fig. 3).
 click to enlarge
Fig. 3: Internalization of the Tac-CFTR chimeras is clathrin dependent.
click to enlarge
Fig. 3: Internalization of the Tac-CFTR chimeras is clathrin dependent.
To delineate the potential sorting signals, alanine substitution was performed in the C-terminal tail of CFTR (Y1424A, L1430A, L1431A). Steady state distribution of COS-1 cells transiently expressing the mutant TacT-Ct demonstrated a tremendous redistribution of TacT-Ct from the endosomal compartment to cell surface, identifying one of the endocytic sorting signals in the cytoplasmic tails of CFTR (Fig. 4).
Fig 4: TacT-chimera internalization is mediated by endocytic sorting signals.
Discussion and Conclusion
Conclusions
The study demonstrated that CFTR undergoes efficient constitutive clathrin-dependent internalization. This process may be directed by the endocytic sorting signals in the cytoplasmic tails, which confer efficient internalization to the Tac reporter. Protein phosphorylation by cAMP-dependent protein kinase A and by protein kinase C may regulate CFTR distribution by promoting its translocation from intracellular compartment to plasma membrane and by inhibiting its internalization. These results suggest that CFTR internalization participate in the determination and regulation of CFTR chloride channel density and consequently of the cAMP-stimulated chloride conductance of the plasma membrane.
References
- Prince, L. S., Workman, R. B., Jr., and Marchase, R. B. (1994) Proceedings of the National Academy of Sciences of the United States of America 91 (11), 5192-6.
- Lukacs, G. L., Segal, G., Kartner, N., Grinstein, S., and Zhang, F. (1997) Biochemical Journal 328 353-61.
- Tousson, A., Fuller, C. M., and Benos, D. J. (1996) Journal of Cell Science 109 (Pt 6), 1325-34.
- Howard, M., DuVall, M. D., Devor, D. C., Dong, J. Y., Henze, K., and Frizzell, R. A. (1995) American Journal of Physiology269 (6 Pt 1), 1565-76.
- Howard, M., Jilling, T., DuVall, M., and Frizzell, R. A. (1996) Kidney International 49 (6), 1642-8.
- Jia, Y., Mathews, C. J., and Hanrahan, J. W. (1997) Journal of Biological Chemistry 272 (8), 4978-84.
- Mohamed, A., Ferguson, D., Seibert, F. S., Cai, H. M., Kartner, N., Grinstein, S., Riordan, J. R., and Lukacs, G. L. (1997) Biochemical Journal 322 (Pt 1), 259-65.
- Lehrich, R. W., Aller, S. G., Webster, P., Marino, C. R., and Forrest, J. N., Jr. (1998) Journal of Clinical Investigation 101 (4), 737-45.
- Lukacs, G. L., Chang, X. B., Kartner, N., Rotstein, O. D., Riordan, J. R., and Grinstein, S. (1992) Journal of Biological Chemistry 267 (21), 14568-72.
| Discussion Board | Previous Page | Your Symposium |