Immunology & Immunological Disorders Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
CTLs play an essential role in cellular immunity in rejection of foreign invaders and tumors by recognizing antigenic peptide in association with major histocompatibility complex (MHC)[1]. Previous reports suggest that antigens in particulate form can elicit CD8+ MHC class I-restricted CTLs response[2]. On the other hand, soluble antigens elicit responses of CD4+ MHC class II-restricted lymphocytes that are usually classified into helper-type T cells[3]. The CD4+ helper T cells then transfer their immunogenic information to B lymphocytes to stimulate the humoral response, i.e., the antibody production. Therefore, at least in vitro, direct induction of CTLs against soluble antigens has been thought difficult except the case in which antigenic peptide was loaded to the antigen presenting dendritic cells[4].
However, studies have also reported that macrophages can induce efficiently CTLs response in vitro when particulate carriers are used to deliver antigenic short peptides (but not longer peptides) and whole protein antigen[5]. These reports indicated that the exogenous particulate antigenic peptides are processed and presented by the macrophages to unprimed CTLs. If autologous CTLs specific to tumor cells that produce soluble- and/or membrane-bound antigen could be induced, they will have strong implications in the development of adoptive immunotherapy of the tumor patient.
Carcinoembryonic antigen (CEA) is a well-known soluble tumor marker frequently detectable in peripheral blood of carcinoma patients. A recombinant vaccinia virus expressing CEA has been shown to be able to induce humoral and, simultaneously, cell-mediated anti-CEA immune responses in mice[6]. Human CTLs response against CEA-producing cells was observed in the patients injected with the recombinant vaccinia-CEA vaccine[7].
We have investigated whether it is possible to induce in vitro human CTLs responding to the CEA-producing cancer cells by utilizing fixed macrophage-rich peripheral blood cells pre-loaded with particulated form of CEA whole protein. Results suggest that the CTLs recognized HLA-A2402 restricted peptides derived from CEA.
Materials and Methods
Target cells and culture condition. We selected three cell lines with the same MHC-class I subtype HLA-A2402, namely, a gastric adenocarcinoma cell line MKN45 that is poorly differentiating and well known to produce a high level of CEA and, as controls, a stomach adenocarcinoma cell line GT3TKB and kidney adenocarcinoma cell line Hpt.10. All the cell lines were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) in an atmosphere of humidified 5% CO2 in air.
Preparation of antigen presenting cells and pulsing CEA-beads. Human PBMC were prepared by the conventional Ficoll-Paque centrifugation method from heparinized peripheral blood of a healthy volunteer who has been identified as carrying HLA-A-2402 subtype. The cells were washed once with PBS, then once with RHAMa medium supplemented with 5% autologous plasma and centrifuged by centrifugation at 1400 rpm for 10 min at room temperature as previously described[8]. The adherent cells with monocytes/macrophages-like morphology were further cultured overnight in the 24-well plate with 2 ml of the medium containing CEA-beads (50ug). As controls, CEA protein alone (50ug/ml), none, or 50 ug of naked latex beads was used. After 24 hr incubation, the adherent cells were fixed with 10% (v/v) formalin in PBS for 1 hr at room temperature and washed thoroughly with the culture medium before the induction of CTLs.
Induction of CTLs from PBMC. On the fixed adherent cells, PBMC (1000000 cells) freshly prepared from the same volunteer were added with 2 ml of RHAMa medium supplemented with human interleukin(IL)-1beta (Otsuka Pharmaceutical Co., Ltd., 167 U/ml), IL-2 (Shionogi & Co., Ltd., 67 U/ml), IL-4 (Ono Pharmaceutical Co., Ltd, 67 U/ml), IL-6 (Ajinomoto, Co., Inc., 134 U/ml), and 5%(v/v) autologous plasma. The induction culture was continued for 4 weeks with half volume of medium change every other day. The effector cells were re-stimulated weekly to a total of 3 re-stimulation at an effector: APC ratio of 2. The fixed adherent cells were renewed by transferring the cultured PBMC once a week. In addition, CEA-peptide specific CTLs were generated by re-stimulating the PBMC 3 times with the fixed adherent cells previously pulsed with CEA652(9) peptide (TYALFVSNL, 50 ug/ml) for 1 hr at 37 ˇC.
Cytotoxicity assay. The target cells, 1000 cells/well in 200ul RPMI 1640 medium containing 5% FBS, were seeded in each well of 96-well plates and were precultured for 12 hrs. The cultured target cells were washed once with PBS(-), then, the cultured PBMC suspended in 200ul of RHAMa containing 5% autologous plasma were added as effector cells to each well at the indicated effector to target ratio (E/T ratio). The cells were cocultured for 24 hrs.
Percentage of surviving target cells was defined as follows:
Surviving target cell(%)=(A-C)/(B-C)x100
where A is the OD570 of the well containing the target cells and the CTLs, B is the OD570 of the 100% control well of the target cells, and C is OD570 of the well containing medium alone.
Inhibition of the cytotoxic activity of the cultured lymphocytes with monoclonal antibodies. Effector cells were pretreated with monoclonal antibodies against CD3, CD8, or CD4 as described previously[9]. These antibodies were used at a final concentration of 5 ug/ml. Target MKN45 cells were precultured overnight in 96-well plates at 10000 cells/well. These target cells were pretreated also with antibodies against human MHC-class I or MHC-class II at a final concentration of 10 ug/ml and then incubated with the effector cells at 37 ˇC for 24 hr.
The inhibition of cytotoxic activity was calculated according to following formula:
% inhibition = (A-B)/(T-B) x 100
where A is OD570 value of the target cells to which the effector cells were added, in which the target cells or the effector cells were pretreated with a monoclonal antibody; B is OD570 value of the target cells to which the effector cells were added (both of the cells were not pretreated with any monoclonal antibodies; and T is OD570 value of the target cells to which neither CTL nor antibodies were added (OD570 of 100% control).
Results
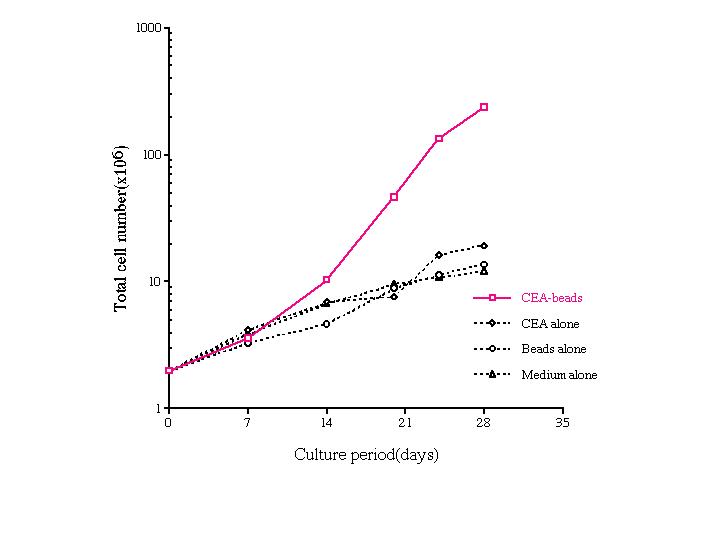 Fig. 1. Cumulative growth curves of the cultured lymphocytes.
Fig. 1. Cumulative growth curves of the cultured lymphocytes.
The cells were cultured with fixed adherent cells pre-loaded with CEA-beads, control CEA protein, naked control beads, or no beads (medium alone).
After 14 days, the number of lymphocytes increased on the fixed cell layer previously loaded with the CEA-beads, but only slightly on the fixed cells previously loaded with control CEA protein and control naked beads as well as medium alone.
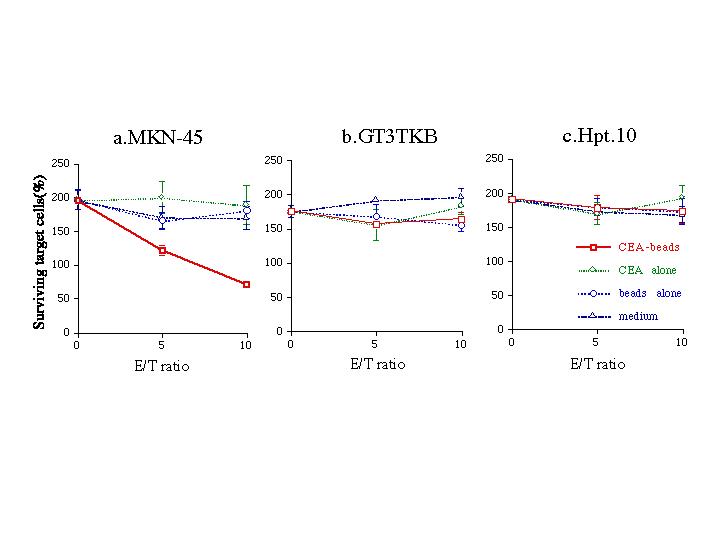 Fig. 2. Cytotoxicity of the effector cells against CEA-producing and non-producing cancer cells.
Fig. 2. Cytotoxicity of the effector cells against CEA-producing and non-producing cancer cells.
The killing assay was performed for 24 hr at E/T ratios indicated. After the coculture of the effector cells with target carcinoma cells, the lymphocytes were gently washed out, then the adhering (therefore conceivable to be surviving) target carcinoma cells were fixed and stained with 0.4% crystal violet and quantified. Note that OD570 of the target carcinoma cells at the start of the coculture was taken as 100%. Also note that the carcinoma cells cultured at the E/T of 0 grew vividly for the 24 hr incubation and, therefore, showed more than 200% of surviving. a, MKN45, CEA positive HLA-A2402 gastric adenocarcinoma cells. b, GT3TKB, CEA negative HLA-A2402 gastric carcinoma cells. c, Hpt.10, CEA negative HLA-A2402 renal carcinoma cells. Each point represents mean of four replicates accompanied with an error bar of SD.We have confirmed expression of CEA and MHC-class I molecules on the surface of the cell lines MKN45 (expressing HLA-A2402, B5201, Cw1202), GT3TKB (HLA-A2402, B5201, Cw1202), and Hpt.10 (HLA-A0206/2402, B3901/5401, Cw0102/0702).
When the lymphocytes were tested for their cytotoxic activity on live target carcinoma cells 7 days after the last re-stimulation, apparent killing was observed only in the combination that contained the lymphocytes stimulated with the fixed adherent cells previously loaded with the CEA-beads and the CEA-producing MKN45 cells (Fig. 2a). In contrast, the fixed adherent cells previously loaded with or without control beads as well as control CEA protein alone did not generate the killing response of the lymphocytes against CEA-producing MKN45 cells. To the other two carcinoma cell lines, GT3TKB and Hpt.10, the lymphocytes did not show any apparent cell killing activity (Fig. 2b and c), although the target cells expressed the common HLA-A2402 on their surface.
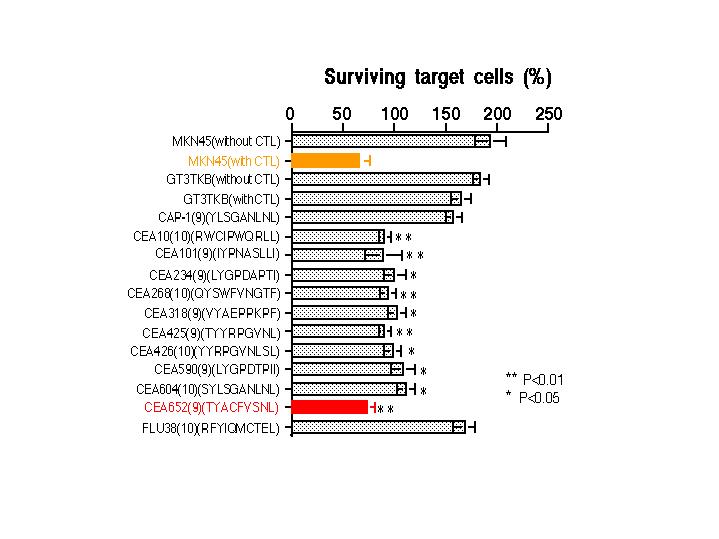 Fig. 3. Cytotoxicity of the CTLs against CEA peptide-pulsed cancer cells.
Fig. 3. Cytotoxicity of the CTLs against CEA peptide-pulsed cancer cells.
GT3TKB cells carrying HLA-A2402 were incubated in the presence of a HLA-A2402-binding peptide derived from CEA at a final concentration of 50 µg/ml for 1 hr and submitted to the killing assay with the CTLs. Each peptide sequence was shown in the parentheses of the fifth line or lower. FLU38(10) is an irrelevant control peptide from a nucleoprotein of influenza virus. CAP-1 (571-579, YLSGANLNL) is the HLA-A2 restricted CTL epitope. Control target cells were GT3TKB cells pulsed no peptides. MKN45 cells were adopted as the CEA-positive control target cells. The killing assay was performed for 24 hr at an E/T ratio of 10. Each bar represents mean of four replicates accompanied with an error bar of SD. Statistically significant differences were found between the bar of the control irrelevant peptide, FLU38(10), and each bar of CEA peptides (P<0.01 or P<0.05).
As shown in Fig. 3, pulse of the 9-mer peptide CEA652(9) (TYACFVSNL) resulted in the same killing as that observed in the CEA producer MKN45 cells. The other 8 peptides revealed lesser antigenic activity than these two peptides in the killing assay, while the control irrelevant peptide, FLU38(10) (RFYIQMCTEL), derived from a nucleoprotein of an influenza virus, showed no antigenic activity to the CTLs (P<0.01 or P<0.05 for target cells pulsed with each CEA peptide compared with target cells pulsed with the irrelevant control peptides, FLU38(10), according to the StudentŐs t-test). Furthermore, no killing activity of the CTLs was observed against the target cells that pulsed with CEA-derived HLA-A2(but not HLA-A2402)-restricted CAP-1, the peptide 571(9) (YLSGANLNL).
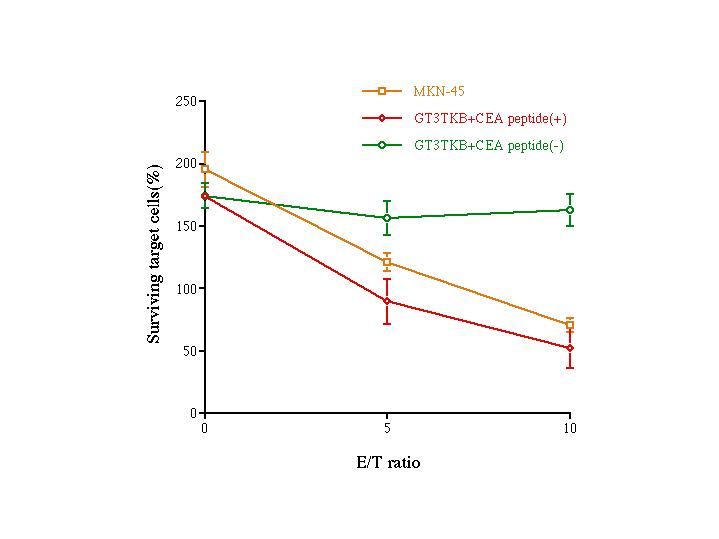 Fig. 4. Killing activity of the CTLs generated on CEA652(9) pulsed and fixed adherent cells from HLA-A2402 donor.
Fig. 4. Killing activity of the CTLs generated on CEA652(9) pulsed and fixed adherent cells from HLA-A2402 donor.
The HLA-A2402 PBMC were stimulated on autologous fixed adherent cells previously pulsed with CEA652(9) (TYALFVSNL) peptide (50 µg/ml) for 1 hr. Cytotoxicity of the generated CTLs was assayed by the crystal-violet staining method (see Materials and Methods or the legend of Fig. 2). Control target cells were GT3TKB cells without CEA 652(9) peptide pulse. Each bar represents mean of four replicates accompanied with an error bar of SD.
The PBMC and the adherent cells used in this experiment were from the same healthy donor carrying HLA-A-2402. The CTLs could kill CEA652(9)-pulsed GT3TKB cells as well as MKN 45 tumor cells that were expressing the CEA antigen, but not the GT3TKB cells that were not pulsed with the CEA peptide.
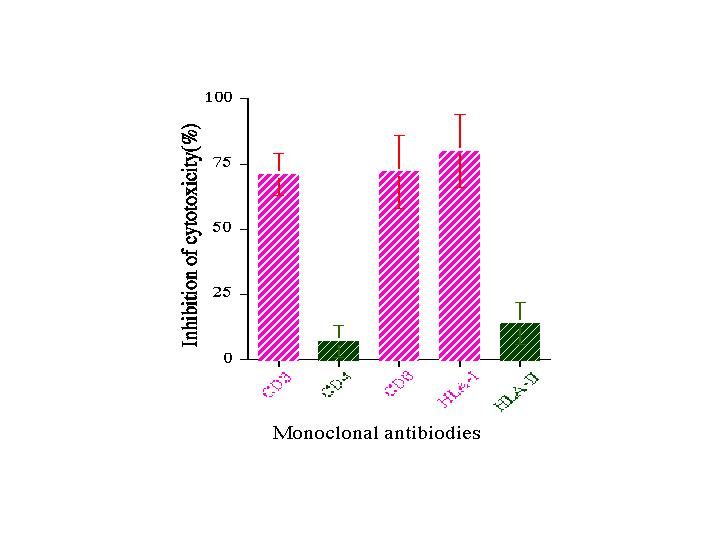 Fig. 5. Inhibition of cytotoxicity of the CTLs with monoclonal antibodies.
Fig. 5. Inhibition of cytotoxicity of the CTLs with monoclonal antibodies.
Inhibition assays were carried out at an E/T ratio of 10. The effector CTL were pretreated with an indicated anti-CD monoclonal antibody at 4 ˇC for 4 hr. Target MKN45 cells were pretreated with an antibody against MHC-class I or MHC-class II molecules at 37 ˇC for 4 hr. The effector cells and the target cells were cocultured for 6 hr at 37 ˇC. Each point represents mean of four replicates accompanied with an error bar of SD.
As shown in Fig. 5, inhibition of the killing activity of the CTLs generated on the fixed adherent cells loaded with CEA-beads was observed on the target MKN45 cells when the lymphocytes were treated with monoclonal antibodies against CD3, CD8 and MHC-class I molecules just before the CV staining assay for 24 hr. In contrast, the killing was not, or to the low extent considerable to be substantially non specific, blocked by anti-CD4 and anti-MHC-class II antibodies.
Discussion and Conclusion
The present results suggest that the cultured lymphocytes on the fixed adherent blood cells pre-loaded with CEA-beads contain MHC class I-restricted CD8+ CTLs (Fig. 5) specific to CEA-producing carcinoma cells (Fig. 2a), but not, or at very low extent, to CEA-non-producing HLA-A2402 carcinoma cells (Fig. 2b and c). MHC- class II molecules were not involved in the cytotoxic response of the CTLs (Fig. 5). The effector cells cultured on the fixed adherent cells pre-loaded with CEA protein alone showed no specific cytotoxicity on any target cells (Fig. 2). These observations provide evidence that the exogenous antigen on the latex beads was processed and presented by MHC-class I molecules after delivery into cells[10].
We also demonstrated here that the CTLs recognized efficiently the CEA epitope peptide TYACFVSNL (CEA652(9)) and less efficiently other peptides so far tested (Fig. 3). CEA652(9) contains two anchor-motif amino acid residues, Y and L with the space of 6 amino acid residues, but the binding affinity to the HLA-A2402 molecule was the third in the tested 11 peptides [K. Takesako and I. Nukaya, personal communication].
Since the present CTL induction was carried out strictly under autologous condition as the lymphocytes were cultured for weeks on the autologous adherent blood cells that previously phagocytized CEA-latex beads and then were fixed with formalin, the results strongly imply that the technique described here will be able to induce autologous CTLs of the tumor-bearing patients against the CEA producing autologous cancer cells in vitro.
We also demonstrated that loading of the CEA peptide to the adherent cells resulted in the generation of the CTLs in vitro (Fig. 4). By this approach, however, so many trials must be made to identify the real epitope peptide among the candidate peptides. Our present approach is simple since CEA whole protein was used as the antigen and previous selective culture of dendritic cells that are hard to proliferate was not required. Fixed antigens on the adherent PBMC will provide long lasting stimulating effect on the expansion of CTLs[11]. Consequently, however, the CTLs responded polyclonally to the CEA epitope peptides (Fig. 3).
We also consider that, if any specific tumor antigens are available and their epitope peptides are unknown, the technique described here will be useful for the induction of autologous CTLs specific to the target tumor antigen even if the target cancer cells are not available.
Acknowledgment. This work was partly supported by the Special Coordination Fund from the Science and Technology Agency of Japan.
References
- Babbit BP, Allen PM, Matsueda G, Habre E, Unanue ER (1985) Binding of immunogenic peptides to Ia histocompatibility molecules. Nature 317: 359
- Falo Jr LD, Kovacsovics-Bankowski M, Thompson K, Rock KL (1995) Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nature Med 1: 649
- Rock KL, Clark K (1996) Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen MHC class I presentation pathway. J Immunol 156: 3721
- Nair S, Zhou F, Reddy R, Huang L, Rouse BT (1992) Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J Exp Med 175: 609
- De Bruijn MLH, Jackson MR, Peterson PA (1995) Phagocyte-induced antigen-specific activation of unprimed CD8+ T cells in vitro. Eur J Immunol 25: 1274
- Kantor J, Irvine K, Abrams S, Kaufman H, Dipierto J, Schlom J (1992) Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst 84: 1084
- Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J (1995) Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst 87: 982
- Liu SQ, Shiba R, Kim BS, Saijo K, Ohno T (1994) Long-term serum/plasma free culture of human cytotoxic T lymphocytes induced from peripheral blood mononuclear cells. Cancer Immunol Immunother 39: 279
- Liu SQ, Saijo K, Todoroki T, Ohno T (1995) Induction of human autologous cytotoxic T lymphocytes on formalin-fixed and paraffin-embedded tumour sections. Nature Med 1: 267
- Harding CV, Song R (1994) Phagocytic processing of particulate antigen by macrophages for presentation by class I MHC molecules. J Immunol 153: 4925
- Horiuchi K, Tsurushima H, Kim BS, Liu SQ, Saijo K, Saijo Y, Nukiwa T, Nomura N, Matsumura M, Ohno T (1998) Expansion of human autologous cytotoxic T lymphocytes on fixed target tumor cells. Cytotechnol in press
| Discussion Board | Previous Page | Your Poster Session |