Invited Symposium: Neuronal Histamine Systems and Behavior
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
INTRODUCTION
Histaminergic neurons are exclusively located in the posterior hypothalamus, specifically in the tuberomammillary nucleus (TM), from where they project diffusely to many parts of the brain, from the olfactory bulb to the spinal cord. In most brain areas histamine is released from varicosities, mostly at non-synaptic sites, indicating modulatory functions similar to those found for other biogenic amines [26]. The diverse actions of the histaminergic neuronal system appear to be mediated by three classes of receptors (H1, H2 and H3).
Recent research emphasis has been placed on the possible role of neuronal histamine in the control of the waking state [49,68], in autonomic [20] and neuroendocrine processes [42], in motivated behaviors like feeding and drinking [44,81], in the regulation of seizure susceptibility [93,94] and in neuropsychiatric disorders [52,55,57]. Furthermore, histamine was found to promote survival of developing hippocampal tissue [11] and to alleviate neuronal damage induced by ischemia [1] and may therefore also be important for processes governing neurogenesis and functional neural recovery.
This review focuses on experiments which investigated a possible involvement of the histaminergic neuronal system in neural plasticity, reinforcement and memory functions. The outcome of these studies suggests that the brain histamine system is a) involved in neural plasticity and functional recovery following unilateral damage of the brain and b) functions as an inhibitory neurochemical substrate in the control of reinforcement and mnemonic processes.
THE ROLE OF THE TM IN NEURAL PLASTICITY
Swanson [78] was one of the first who reported extrahypothalamic projections of the TM. Specific crossed and uncrossed projections from the TM to the caudate putamen were then described by Watanabe et al. [86] and by Steinbusch et al. [72]. We confirmed these findings and demonstrated neuroplastic changes in tuberomammillary-striatal projections in relation to recovery from behavioral asymmetries induced by hemivibrissotomy [88] and unilateral 6-hydroxydopamine (6-OHDA) lesion of the substantia nigra (SN).
 Click to enlarge
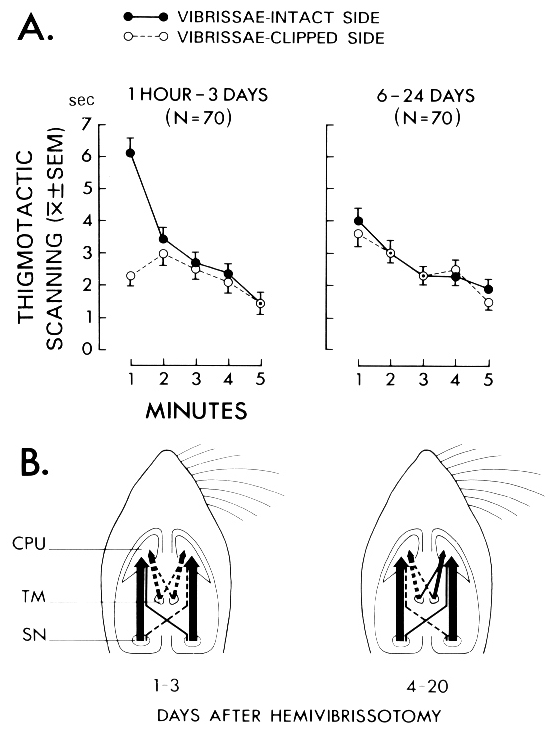
Fig. 1: (A) Duration (s) of thigmotactic scanning with the vibrissae-intact side (continuous line) and the vibrissae-clipped side (broken line) through a 5-min test session. Rats tested between 1 h and 3 days after hemivibrissotomy exhibited a strong asymmetry in scanning during the first minute of testing, as they scanned more with the vibrissae-intact side (left). This behavioral asymmetry was absent in rats tested 6 days, or later, after clipping the vibrissae (right). (B) Corresponding neuronal changes in the tuberomammillary-striatal projections 1-3 days (left) and 4-20 days after hemivibrissotomy (right) in comparison with the findings in the nigro-striatal projections. (Data from [33,88].)
Click to enlarge
Fig. 1: (A) Duration (s) of thigmotactic scanning with the vibrissae-intact side (continuous line) and the vibrissae-clipped side (broken line) through a 5-min test session. Rats tested between 1 h and 3 days after hemivibrissotomy exhibited a strong asymmetry in scanning during the first minute of testing, as they scanned more with the vibrissae-intact side (left). This behavioral asymmetry was absent in rats tested 6 days, or later, after clipping the vibrissae (right). (B) Corresponding neuronal changes in the tuberomammillary-striatal projections 1-3 days (left) and 4-20 days after hemivibrissotomy (right) in comparison with the findings in the nigro-striatal projections. (Data from [33,88].)
Hemivibrissotomy, which stands for the removal of the tactile hairs (vibrissae) on one side of the rat's snout, induces a transient asymmetry in the side of the face used to scan the wall while traversing the edge of an open field (i.e., 'thigmotactic scanning'; see Fig. 1A) from which rats recover over time [33]. Time-related to these behavioral changes we found an increase in "strength" (i.e., in structure and/or activity) in the uncrossed and crossed projections from the TM to the caudate nucleus. Rats examined four to twenty days after unilateral clipping of vibrissae had more HRP-labeled cells in the crossed and uncrossed projections from the TM nuclei to the caudate nucleus on the side of intact vibrissae (i.e., to the hemisphere deprived of vibrissal sensory input) compared to projections to the caudate nucleus on the side of vibrissae removal (Fig. 1B). The neuronal asymmetries in the TM-striatal projections were in the same direction as the asymmetries previously found in nigro-striatal projections after hemivibrissotomy [74,75]. However, unlike in the nigro-striatal pathway, apparent neuronal asymmetries in the tuberomammillary-striatal projections were only evident during the time period when the rats had recovered from the behavioral asymmetry. Given the coincidence of changes in striatal afferents from the SN and from the TM, it is conceivable that an interaction between histamine and dopamine (DA) could play a role in the control of recovery of function. In line with this suggestion, nigrostriatal DA denervation was found to induce a marked up-regulation of H3-receptors in the striatum, which was reduced by dopamine D1 receptor stimulation [61]. Taken together, these data provided first evidence for a role of the tuberomammillary-striatal system in behavioral plasticity subsequent to unilateral removal of the vibrissae, in concert with the nigro-striatal system.
Based on these anatomical findings we were interested in a behavioral correlate of a lesion in the TM region. Therefore, we investigated the influence of a unilateral direct current (DC) lesion in the TM region on thigmotactic scanning behavior. Destruction of the TM region was found to produce more thigmotactic wall scanning behavior with the vibrissae contralateral to the lesion; the histamine precursor histidine reversed the effects of the TM lesion, suggesting that histamine is involved in this effect [89]. In sharp contrast, a unilateral 6-OHDA lesion of the SN produced more wall scanning behavior with the vibrissae ipsilateral to the lesion [73]. The finding that lesions in the SN and TM have opposite effects on scanning behavior suggests that the projections (perhaps to the striatum) could represent a reciprocally acting regulatory system in terms of sensorimotor processes, possibly involving DA and histamine. In accordance with the idea of a reciprocal relationship between the TM system (histaminergic) and the SN (dopaminergic) is the finding that functional recovery from a unilateral 6-OHDA lesion of the SN was associated with an enhancement of the nigro-striatal projections [50], whereas in rats that failed to recover from the nigral lesion, an enhancement of the TM-striatal projections (based on the extent of HRP-labeling in TM and SN after HRP injection into the caudate-putamen region) was observed [51]. These findings suggest that the increase in HRP-labeling seen in the tuberomammillary-caudate projections indicates an enhancement of histaminergic activity, which, in turn may be related to the lack of recovery from a unilateral SN lesion, and to the increase in asymmetry that develops over time in such animals.
HISTAMINE, REINFORCEMENT AND MEMORY
REINFORCEMENT A number of pharmacological studies have examined the role of histamine in reinforcement processes. For example, the self-administration of histamine and histamine-blocking compounds has been evaluated [62]. The injection of histamine and histamine antagonists was also studied in combination with rewarding brain stimulation [10,60,82,87]. Their effects were assessed on operant behavior [4,47,79] and in conditioned place preference tasks, either alone [46] or in combination with stimulants [45] and opioids [77]. The results of these experiments provided evidence that histamine agonists may have aversive properties, whereas histamine antagonists, particularly those blocking the H1-receptor, can exert reinforcing as well as reward potentiating effects. The TM nucleus itself has largely been neglected in the search for the neural mechanisms underlying reinforcement. Some studies, in which the hypothalamic region was mapped for reinforcing properties of electrical stimulation reported negative or ambivalent stimulation effects in the posterior hypothalamus, the region where the TM is located [53]. Given the evidence for a role of the TM projections in neural plasticity and functional recovery and the proposed reciprocal relation between histaminergic and dopaminergic mechanisms (see above), we performed a series of experiments to examine the possible involvement of the TM in the brain's reinforcement system.
 Click to enlarge
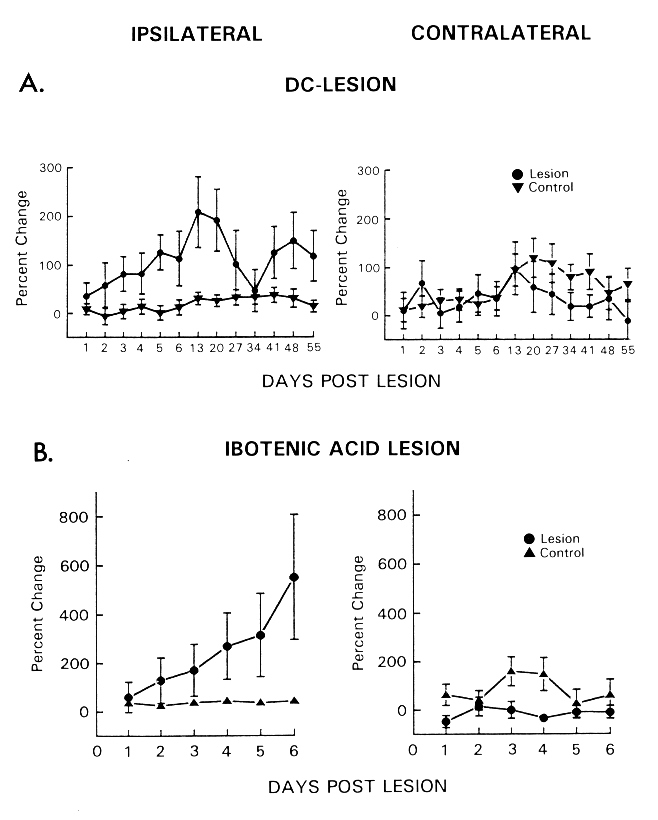
Fig. 2: Lateral hypothalamic self-stimulation ipsi- (left) and contralateral (right) to the side of a unilateral DC (A) or ibotenic acid lesion (B) in the region of the tuberomammillary nucleus. Response rates are expressed as mean (with S.E.M.) percentage of corresponding baseline values. (Data from [84,85].)
Click to enlarge
Fig. 2: Lateral hypothalamic self-stimulation ipsi- (left) and contralateral (right) to the side of a unilateral DC (A) or ibotenic acid lesion (B) in the region of the tuberomammillary nucleus. Response rates are expressed as mean (with S.E.M.) percentage of corresponding baseline values. (Data from [84,85].)
In the first experiment the effects of a unilateral DC lesion in the rostroventral part of the TM (E2-region) on lateral hypothalamic self-stimulation were assessed [84]. From the second day post-lesion, we found a gradual increase in response rate in TM-lesioned animals, which peaked on day thirteen in the ipsilateral hemisphere only. Response rates remained elevated during the following seven weekly tests (Fig. 2A). Lateral hypothalamic self-stimulation was also examined following a unilateral lesion of the TM with ibotenic acid, an excitatory amino acid that destroys neurons and leaves fibers of passage intact [6,65]. We hypothesized that, if the intrinsic neurons of the TM region were responsible for the potentiating effects of the electrolytic lesion, the same effect should result from a neurotoxic insult which spares fibers of passage [85]. Figure 2B shows increasing response rates obtained from the hemisphere ipsilateral to the TM lesion. Since the response curves revealed in both studies were very similar, it can be concluded that the destruction of TM-intrinsic neurons was critical for the effects, rather than the denervation of remote structures induced by damaged fibers of passage. Furthermore, it is important to note that in both experiments facilitation of self-stimulation only occurred after destruction of the E2- but not of the E1-subgroup of the TM. This dissociation can be considered as the first indication for a functional specificity of a cell population within the TM, which until now has been defined on anatomical grounds only.
To establish whether the observed effects following TM lesions are mediated by histamine, pharmacological studies were performed to investigate the effects of different histamine-receptor blocking drugs in the nucleus accumbens (NAc) and in the nucleus basalis magnocellularis (NBM) region. Both structures are known to play an important role in reinforcement processes [31,43], receive specific histaminergic input [56,72] and contain all three subtypes of histamine receptors [66]. The administration of the histamine antagonists was either combined with lateral-hypothalamic self-stimulation or their effects were examined with the corral version of the conditioned place preference paradigm ([27] for details). In the NAc, the administration of 10 microgram of the H1-blocking drug chlorpheniramine produced a lateralized increase of hypothalamic self-stimulation and was effective in inducing a conditioned corral preference, indicative of a positively reinforcing action. Furthermore, the effects of chlorpheniramine were found to be restricted to the caudal part of the NAc since injection of the H1-antagonist into the rostral NAc did not affect the behavior in either paradigm [34]. In the nucleus basalis magnocellularis region, chlorpheniramine as well as the H2-antagonist ranitidine were tested for possible reinforcing effects by the use of the corral method [58]. A single injection of chlorpheniramine of 10 or 20 microgram into the NBM increased the time the animals spent in the corral previously paired with the drug treatment, indicative of a reinforcing action of the H1-antagonist. In contrast, the H2-antagonist ranitidine did not significantly influence the preference behavior within the entire dose range tested (0.1 to 20 microgram).
Taken together, the outcome of these studies suggests that the TM and its histaminergic projections exert inhibitory effects on reinforcement under normal conditions. Reducing histaminergic activity either by a partial destruction of TM-intrinsic histamine neurons or by inhibiting the histaminergic transmission at H1-receptive sites apparently results in a disinhibition of reinforcement. The described inhibitory function of the TM in the control of intracranial self-stimulation and the effects of histaminergic agonists and antagonists on various measures of reinforcement stand in sharp contrast to the effects of DA on reinforcement. It is widely accepted that DA agonists facilitate and DA antagonists inhibit brain stimulation reward [91]. Thus, DA seems to influence the brain's reinforcement system in a way which is again reciprocal to histamine. The brain's reinforcement mechanism or mechanisms can be considered as being activated in a tonic fashion by DA and histamine, with the further promoting, and the latter diminishing reinforcement, i.e., the effectiveness of a reinforcing stimulus to increase the probability of recurrence of a preceding operant behavior, as evidenced by changes in the organism's degree of "preference for" that stimulation or for place cues that have been associated with such reinforcing stimulation.
LEARNING AND MEMORY PROCESSES
The role of the histaminergic system in learning and memory has been generally investigated with contradictory results ([54] for review). For example, histamine was reported to improve inhibitory and active avoidance conditioning [12,40], whereas administration of H1-antagonists disrupted learning in an active avoidance task [36,39]. Thioperamide, a histamine H3-antagonist, was found to improve the retention performance of adult [76] and senescence-accelerated mice [48], whereas the H3-agonist imetit produced a dose-dependent disruption [76]. Furthermore, histamine was reported to improve memory retrieval in old and hippocampus-lesioned rats [37,38]. On the contrary, histamine has been shown to reduce active avoidance responding, an effect mediated via the H1-receptive site [80], and long-term depletion of neuronal histamine by alpha-fluoromethylhistidine was shown to improve active avoidance conditioning [8]. Microinjection of histamine into the dentate area and subiculum complex adversely affected active avoidance conditioning via histamine H1-receptive sites [2]. The H3-receptor agonist (R)-alpha-methylhistamine was found to improve navigation performance in the water maze [69]. The reasons for these discrepant findings require clarification. Moreover, the exact functions of the histamine receptor subtypes still remain to be determined. For example, mutant mice lacking the H1-receptor showed reduced aggressive and exploratory behaviors but no apparent change in learning capacities [92]. Furthermore, although functionally characterized as an inhibitory autoreceptor, the histamine H3-receptor is not restricted to presynaptic elements of the histaminergic neurons. It also functions as a heteroreceptor, e.g., modulating the release of serotonin [63] or noradrenaline [64].
Given the parallelism between reinforcing and memory-promoting effects of manipulations of the brain [32], we hypothesized that lesion of the TM region could have a facilitatory effect on learning and mnemonic processing in addition to its facilitatory effect on reinforcement processes. Furthermore, we asked whether TM lesions might exert a beneficial action on the performance of aged rats, which are considered to be an animal model for learning and memory disturbances related to aging and nervous system disorders like Alzheimer's disease [18]. In the first series of experiments [17,41], adult (3-month-old) and aged (31-month-old) rats with a bilateral DC lesion in the TM region were tested along with sham-lesioned controls in a set of learning tasks, which differed in terms of complexity and reward contingencies (habituation of exploratory activity, inhibitory avoidance, discrimination learning).
 Click to enlarge
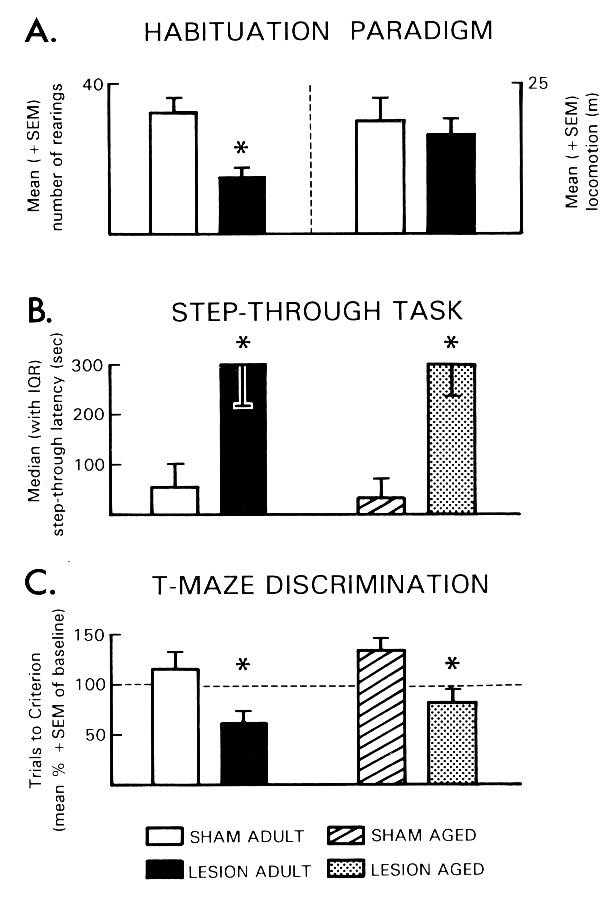
Fig. 3: The effects of a bilateral DC lesion in the TM region on performance of adult (3-month-old) and aged (31-month-old) rats in different learning tasks. (A) Habituation paradigm: Number of rearings (left) and distance traveled in the open field (right) during test for habituation learning. (B) Step-through task: Step-through latencies revealed in the retention test. (C) T-maze discrimination: Number of trials required to reach the criterion of 5 successive correct choices in the T-maze during the retention test. Trials to criterion during retention test are expressed as percentage of corresponding baseline values (= 100%, dashed line); *p < .05 vs. sham-lesioned controls. (Data from [17,41].)
Click to enlarge
Fig. 3: The effects of a bilateral DC lesion in the TM region on performance of adult (3-month-old) and aged (31-month-old) rats in different learning tasks. (A) Habituation paradigm: Number of rearings (left) and distance traveled in the open field (right) during test for habituation learning. (B) Step-through task: Step-through latencies revealed in the retention test. (C) T-maze discrimination: Number of trials required to reach the criterion of 5 successive correct choices in the T-maze during the retention test. Trials to criterion during retention test are expressed as percentage of corresponding baseline values (= 100%, dashed line); *p < .05 vs. sham-lesioned controls. (Data from [17,41].)
Habituation was investigated in a rectangular open field ([19] for details). Adult rats with bilateral DC lesions in the TM region showed enhanced habituation (Fig. 3A). Inhibitory avoidance conditioning was performed using a one-trial step-through avoidance task. As depicted in Fig. 3B, facilitation of retention performance was observed in both adult and aged rats which had received bilateral DC lesions in the TM region. Compared to sham-lesioned controls, TM-lesioned adult and aged rats had longer step-through latencies and showed a greater tendency to remain in the start compartment for the entire test interval. These findings confirm those of a recent experiment that demonstrated facilitation of retention using active avoidance conditioning [67]. In the third experiment of this series, TM-lesioned adult and aged rats were tested on a left/right discrimination in a water-filled T-maze. During the acquisition of the left/right discrimination, TM-lesioned adult and aged rats did not significantly differ from sham-lesioned controls in the number of trials to reach the criterion. During retention test, however, TM-lesioned adult and aged rats displayed improved discrimination performance as indicated by fewer trials needed to reach the learning criterion (Fig. 3C). This series of experiments provided evidence that a bilateral electrolytic lesion of the TM region can improve the performance of adult and aged rats in a variety of learning tasks, including avoidance and escape learning, a discrimination task and a habituation paradigm.
The fact that habituation "learning" was improved is important, since, for one, habituation does not involve application of conventional reinforcers, such as food or escape from or avoidance of aversive stimulation, and secondly, since the TM histaminergic system is thought to play a role in stress, perception of pain and thermoregulation [29,54], which are important factors in aversive conditioning, but not for habituation. This makes an interpretation of the performance enhancement following TM lesion simply in terms of an interaction between the lesion and physiological processes induced by a punishing/aversive stimulus, unlikely. Based on these findings, the objectives of a follow-up study were two-fold [17]. In order to determine whether the facilitation of learning and memory was due to the destruction of intrinsic TM neurons, adult (3-month-old) and aged (28 to 31-month-old) rats with bilateral ibotenic acid lesions of the TM region were tested along with vehicle-injected controls in the Morris water maze in which old rats display marked performance deficits [28]. Furthermore, the number of histamine cells was determined at the site of the neurotoxic lesion by immunohistochemistry using specific antibodies against the amine ([71] for details).
 Click to enlarge
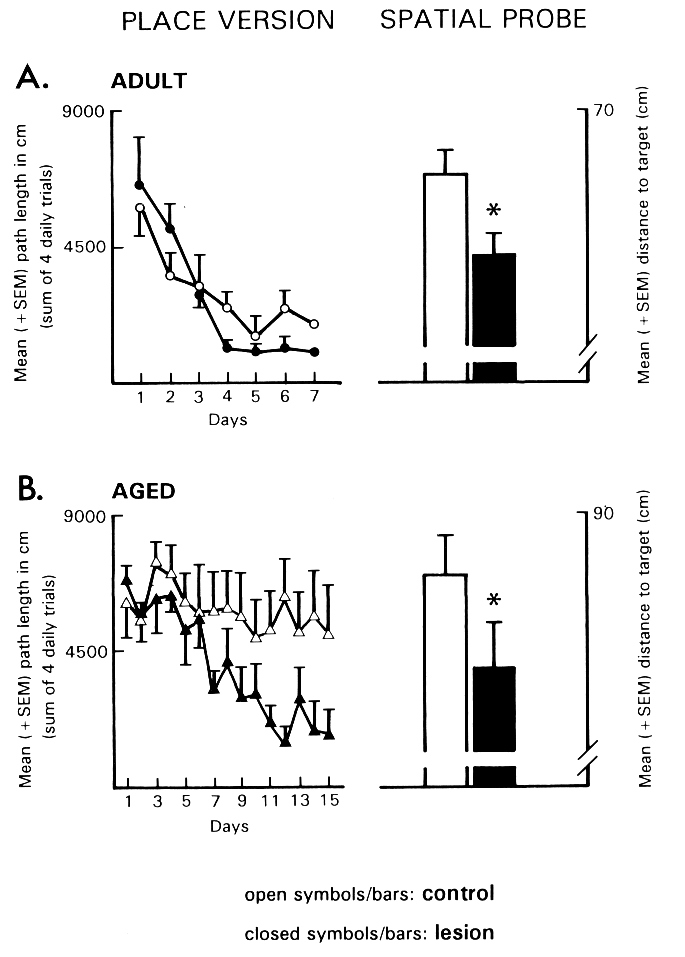
Fig. 4: The effects of a bilateral ibotenic acid lesion in the TM-region on the navigation performance of (A) adult (3-month-old) and (B) aged (28 to 31-month-old) rats in the Morris water maze. Left: Path length to find the hidden platform in the place version of the maze. Right: Distance to target during a spatial probe trial without platform. *p < .05 vs. vehicle-injected controls. (Data from [17].)
Click to enlarge
Fig. 4: The effects of a bilateral ibotenic acid lesion in the TM-region on the navigation performance of (A) adult (3-month-old) and (B) aged (28 to 31-month-old) rats in the Morris water maze. Left: Path length to find the hidden platform in the place version of the maze. Right: Distance to target during a spatial probe trial without platform. *p < .05 vs. vehicle-injected controls. (Data from [17].)
The main finding of this study was that adult and aged rats with neurotoxic lesions of the TM showed accelerated navigation performance in the course of place learning in the maze and an improved ability to locate the platform site during a spatial probe trial (Fig. 4). Inspection of the site of the ibotenic acid microinjection in the TM region revealed a marked decrease of histamine-staining neurons mainly in the rostral part of the TM. Results from pharmacological experiments dealing with the effects of histamine antagonists on avoidance conditioning are also suggestive for an inhibitory action of histamine on mnemonic processes [59]. Possible mnemogenic effects of histamine antagonists were examined with the one trial uphill avoidance task, which involves punishment of a high-probability turning response on a tilted platform ([70] for details). Immediately after the learning trial, that is, after a shock was administered upon performing the response, different doses of chlorpheniramine and ranitidine were injected unilaterally into the NBM region of adult rats. Control groups included vehicle-injected rats and a group of animals given an injection of chlorpheniramine (20 microgram) 5 h after shock administration. Retention testing was performed 24 h after the learning trial. Administration of chlorpheniramine facilitated the retention performance at 10 and 20 microgram but not at lower doses of 0.1 and 1 microgram. In contrast, none of the doses of the H2-antagonist influenced avoidance conditioning.
The failure of the delayed post-trial injection of chlorpheniramine to influence learning indicates that the H1-antagonist influenced learning by modulating memory storage processes rather than by acting on performance variables (e.g., arousal, attention, motor coordination) during acquisition or retrieval of the task. In a further experiment [16], adult (4-month-old) as well as aged (31-month-old) rats received an intraperitoneal injection of chlorpheniramine (5 or 10 mg/kg) immediately after the learning trail on the step-through avoidance task. The post-trial injection of 10 mg/kg chlorpheniramine significantly improved the retention of the aged rats. Furthermore, aged animals treated with 10 mg/kg chlorpheniramine displayed performance scores comparable with those of vehicle-treated adult rats, indicating that the H1-antagonist had reversed the performance deficit observed in the old rats.
The mechanisms by which chlorpheniramine was effective to facilitate inhibitory avoidance conditioning in adult and old rats is open to question. Recent experiments performed with in vivo microdialysis in anesthetized rats revealed that administration of chlorpheniramine can produce increases in extracellular levels of acetylcholine in the cortex and dopamine in the striatum [13,14], suggesting that the memory enhancing as well as reinforcing effects of the H1-antagonist were mediated, at least in part, through cholinergic and dopaminergic mechanisms.
HIPPOCAMPAL SIGNAL TRANSFER
The hippocampus is thought to play an important role in memory formation [15] and in reward-related processes [95]. The hippocampus receives histaminergic fibers through both a ventral and a dorsal route [35] and contains all three subtypes of histamine receptors [3,22]. Furthermore, a number of electrophysiological studies have demonstrated that TM histamine projections are involved in the subcortical modulation of neuronal excitability and synaptic plasticity in the hippocampal circuitry [5,24,26]. Given the evidence for an inhibitory role of the TM in reinforcement and mnemonic processes and the functional link between TM and hippocampus, we [90] gauged whether activation of the TM could modulate evoked field potentials in the dentate gyrus, frequently used to study electrophysiological correlates of learning [7,21]. Therefore, paired-pulses of electrical stimulation were delivered to the perforant path (PP) and evoked field potentials (fEPSPs) were recorded in the dentate gyrus (DG) in freely moving rats. Prior to activating the PP, the TM was triggered by electrical stimulation when the rat explored an unfamiliar environment (Type I, "theta" behavior, including walking, sniffing and rearing according to ref. [83]) or when the animals showed Type II, "non-theta" behavior, including grooming, awake immobility and slow-wave sleep.
The results indicate that activation of the histaminergic TM nucleus in the freely moving rat differentially affected the efficacy of afferent transmission to the hippocampus, depending on the behavioral state of the animal. Prestimulation of the TM was found to modulate neuronal transmission in the PP during learning-related exploratory behavior, but not during "non-theta" related behaviors, including awake immobility. During exploration both the conditioning as well as the test response of the dentate fEPSPs decreased with increasing TM train stimulation intensities, whereas the population-spikes were unchanged. Similar excitability changes in the PP-dentate area were previously observed in hippocampal slices exposed to high concentrations of histamine [23,25].
These results indicate that the TM and the hippocampus may comprise a common system involved in the inhibition of the brain's reinforcement system and suggest that the TM projection system exerts its inhibitory action on associative functioning by interfering negatively with the signal transfer across the PP-granule cell synapses of the dentate gyrus. Congruent with this hypothesis, it was recently found that microinjection of histamine into the dentate area adversely affected active avoidance conditioning [2].
CONCLUSION
Results from this laboratory suggest that TM histamine projections are involved in behavioral asymmetries and in subsequent behavioral recovery after hemivibrissotomy and unilateral 6-OHDA lesions of the SN. Furthermore, our findings indicate that the histaminergic neuronal system (specifically histaminergic fibers arising from the E2-subgroup) may function as an inhibitory neurochemical substrate in the control of reinforcement and mnemonic processes. Both amplification of rewarding hypothalamic stimulation as well as facilitation of mnemonic processes were demonstrated following destruction of TM. On the other hand, electrical or chemical stimulation of the TM was found to negatively interfere with the signal transfer in the hippocampus during learning-related exploratory behaviors. Moreover, administration of the histamine H1-receptor antagonist chlorpheniramine, but not the H2-receptor antagonist ranitidine, was found to exert reinforcing effects and to promote learning in projection areas of the TM known to be crucial for reward and mnemonic functions, namely, the NBM and the NAc.
These results are the first to focus on an inhibitory element in the neural system underlying the reinforcement process ("stamping-in"). Up to now, such an inhibitory substrate has been largely ignored or neglected in the attempt to characterize the neural basis of the reinforcement system [30]. Furthermore, we found that lesions of the TM or blockade of certain histamine receptors generally induced changes in behavioral parameters, which were opposite to those known to occur after destruction or pharmacological manipulations of the SN [9,33]. Such an antagonism was evident for turning and thigmotactic scanning, lateral-hypothalamic self-stimulation, place conditioning and mnemonic functioning. The evidence that the TM and SN and their transmitters DA and histamine can act in a reciprocal fashion with regard to the behaviors investigated so far, may be indicative of a functional link between the tuberomammillary-striatal and the nigrostriatal system.
Acknowledgments. This work was supported by grant Hu 306/11-2 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- Adachi N, Oishi R, Itano Y, Yamada T, Hirakawa M and Saeki K (1993) Aggravation of ischemic neuronal damage in the rat hippocampus by impairment of histaminergic neurotransmission. Brain Res. 602: 165-168.
- Alvarez EO and Banzan AM (1996) Hippocampus and learning. Possible role of histamine receptors. Medicina (B-Aires) 56: 155-160.
- Arrang JM, Garbarg M, Lancelot JC, Lecomte JM, Pollard H, Robba M, Schunack W and Schwartz JC (1987) Highly potent and selective ligands for histamine H3-receptors. Nature 327: 117-123.
- Bergman J and Spealman RD (1986) Some behavioral effects of histamine H1 antagonists in squirrel monkeys. J. Pharmacol. Exp. Ther. 239: 104-110.
- Brown RE, Fedorov NB, Haas HL and Reymann KG (1995) Histaminergic modulation of synaptic plasticity in area CA1 of rat hippocampal slices. Neuropharmacology 34: 181-190.
- Buscher W, Schugens M, Wagner U and Huston JP (1989) Interhemispheric relationship between lateral hypothalamic self-stimulation and the region of the nucleus tegmenti pedunculo-pontinus. Brain Res. 487: 321-334.
- Buzsaki G, Grastyan E, Czopf J, Kellenyi L and Prohaska O (1981) Changes in neuronal transmission in the rat hippocampus during behavior. Brain Res. 225: 235-247.
- Cacabelos R and Alvarez XA (1991) Histidine decarboxylase inhibition induced by alpha-fluoromethylhistidine provokes learning-related hypokinetic activity. Agents Actions 33: 131-134.
- Carey RJ (1982) Unilateral 6-hydroxydopamine lesions of dopamine neurons produce bilateral self-stimulation deficits. Behav. Brain Res. 6: 101-114.
- Cohn CK, Ball GG and Hirsch J (1973) Histamine: Effect on self-stimulation. Science 180: 757-758.
- Curtis M, Bergman H, Price ML, Srivastava N and Granholm AC (1995) Hypothalamic tissue stimulates hippocampal pyramidal neuron survival during development: Evidence from intraocular double transplants. Hippocampus 5: 584-594.
- De Almeida MA and Izquierdo I (1988) Intracerebroventricular histamine, but not 48/80, causes posttraining memory facilitation in the rat. Arch. Int. Pharmacodyn. Ther. 291: 202-207.
- Dringenberg HC, De Souza-Silva MA, Rossmüller J, Huston JP and Schwarting RKW (1998) Histamine H1 receptor antagonists produce increases in extracellular acetylcholine in rat frontal cortex and hippocampus J Neurochem. 70: 1750-1758.
- Dringenberg HC, De Souza-Silva MA, Schwarting RKW and Huston JP (1998) Increased levels of extracellular dopamine in neostriatum and nucleus accumbens after histamine H1 receptor blockade. Naunyn-Schmiedebergs Arch. Pharmacol. (in press).
- Eichenbaum H, Cohen NJ, Otto T and Wible C (1991) Memory representation in the hippocampus: Functional domain and functional organization. In Memory: Organization and Locus of Change, Squire LR, Weinberger NM, Lynch G and McGaugh JL, eds. Oxford University Press, New York, pp. 163-204.
- Frisch C, Hasenöhrl RU, Huston JP (1997) The histamine H1-antagonist chlorpheniramine facilitates learning in aged rats. Neurosci. Lett. 229: 89-92.
- Frisch C, Hasenöhrl RU, Haas HL, Weiler HT, Steinbusch HWM and Huston JP (1998) Facilitation of learning after lesions of the tuberomammillary nucleus region in adult and aged rats. Exp. Brain Res. 118: 447-456.
- Gallagher M and Nicolle MM (1993) Animal models of normal aging: Relationship between cognitive decline and markers in hippocampal circuitry. Behav. Brain Res. 57: 155-162.
- Gerhardt P, Hasenöhrl RU, Hock FJ and Huston JP (1993) Mnemogenic effects of injecting RA-octil, a CE-inhibitor derivate, systemically or into the basal forebrain. Psychopharmacology 111: 442-448.
- Göthert M, Garbarg M, Hey JA, Schlicker E, Schwartz JC and Levi R (1995) New aspects of the role of histamine in cardiovascular function: Identification, characterization, and potential pathophysiological importance of H3 receptors. Can. J. Physiol. Pharmacol. 73: 558-564.
- Green EJ, McNaughton BL and Barnes CA (1990) Exploration-dependent modulation of evoked responses in fascia dentata: Dissociation of motor, EEG, and sensory factors and evidence for a synaptic efficacy change. J. Neurosci. 10: 1455-1471.
- Green JP (1983) Histamine receptors in brain. In Handbook of Psychopharmacology, Vol. 17, Iverson LL, Iverson SD and Snyder SH, eds. Plenum Press, New York, pp. 384-420.
- Greene RW and Haas HL (1990) Effects of histamine on dentate granule cells in vitro. Neuroscience 34: 299-303.
- Haas HL (1992) Electrophysiology of histamine receptors. In The Histamine Receptor, Schwartz JC and Haas HL, eds. Wiley-Liss, New York, pp. 161-177.
- Haas HL and Greene RW (1986) Effects of histamine on hippocampal pyramidal cells of the rat in vitro. Exp. Brain Res. 62: 123-130.
- Haas HL, Sergueeva OA, Vorobjev VS and Sharonova IN (1995) Subcortical modulation of synaptic plasticity in the hippocampus. Behav. Brain Res. 66: 41-44.
- Hasenöhrl RU, Oitzl MS and Huston JP (1989) Conditioned place preference in the corral: A procedure for measuring reinforcing properties of drugs. J. Neurosci. Methods 30: 141-146.
- Hasenöhrl RU, Frisch C, Nikolaus S and Huston JP (1994) Chronic administration of neurokinin SP improves maze performance in aged Rattus norvegicus. Behav. Neural Biol. 62: 110-120.
- Hough LB (1988) Cellular localization and possible functions for brain histamine: Recent progress. Prog. Neurobiol. 30: 469-505.
- Huston JP (1982) Searching for the neural mechanism of reinforcement (of "stamping-in"). In The Neural Basis of Feeding and Reward, Hoebel BG and Novin D, eds. Academic Press, New York, pp. 75-83.
- Huston JP and Hasenöhrl RU (1995) The role of neuropeptides in learning: Focus on the neurokinin substance P. Behav. Brain Res. 66: 117-127.
- Huston JP, Mueller CC and Mondadori C (1977) Memory facilitation by posttrial hypothalamic stimulation and other reinforcers: A central theory of reinforcement. Biobehav. Rev. 1: 143-150.
- Huston JP, Steiner H, Weiler HT, Morgan S and Schwarting RKW (1990) The basal ganglia-orofacial system: Studies on neurobehavioral plasticity and sensory-motor tuning. Neurosci. Biobehav. Rev. 14: 433-446.
- Huston JP, Frisch C, Privou C, Zimmermann PK and Hasenöhrl RU (1996) The role of the histaminergic neuron system in reward and memory processes. Soc. Neurosci. Abstr. 22: 141.
- Inagaki N, Yamatodani A, Ando-Yamamoto M, Tohyama M, Watanabe T and Wada H (1988) Organization of histaminergic fibers in the rat brain. J. Comp. Neurol. 273: 283-300.
- Kamei C and Tasaka K (1991) Participation of histamine in the step-through active avoidance response and its inhibition by H1-blockers. Jpn. J. Pharmacol. 57: 473-482.
- Kamei C and Tasaka K (1992) Effect of intracerebroventricular injection of histamine on memory impairment induced by hippocampal lesions in rats. Jpn. J. Pharmacol. 58 [Suppl.1]: 55.
- Kamei C and Tasaka K (1993) Effect of histamine on memory retrieval in old rats. Biol. Pharm. Bull. 16: 128-132.
- Kamei C, Chung YH and Tasaka K (1990) Influence of certain H1-blockers on the step-through active avoidance response in rats. Psychopharmacology 102: 312-318.
- Kamei C, Okumura Y and Tasaka K (1993) Influence of histamine depletion on learning and memory recollection in rats. Psychopharmacology 111:376-382.
- Klapdor K, Hasenöhrl RU and Huston JP (1994) Facilitation of learning in adult and aged rats following bilateral lesions of the tuberomammillary nucleus region. Behav. Brain Res. 61: 113-116.
- Knigge U, Kjaer A, Larsen PJ, Jorgensen H, Bach FW, Moller M and Warberg J (1995) Effect of histamine on gene expression and release of proopiomelanocortin-derived peptides from the anterior and intermediate pituitary lobes in conscious male rats. Neuroendocrinology 62: 319-325.
- Koob GF and Bloom FE (1988) Cellular and molecular mechanisms of drug dependence. Science 242: 715-723.
- Kraly FS, Tribuzio RA, Keefe ME, Kim YM and Lowrance R (1995) Endogenous histamine contributes to drinking initiated without postprandial challenges to fluid homeostasis in rats. Physiol. Behav. 58: 1137-1143.
- Masukawa Y, Suzuki T and Misawa M (1993) Differential modification of the rewarding effects of methamphetamine and cocaine by opioids and antihistamines. Psychopharmacology 111: 139-143.
- Mattioli R, Nelson CA, Huston JP and Spieler R (1996) Reinforcing properties of chlorpheniramine in goldfish. Soc. Neurosci. Abstr. 22: 141.
- McKearney JW (1982) Stimulant actions of histamine H1 antagonists on operant behavior in the squirrel monkey. Psychopharmacology 77: 156-158.
- Meguro K, Yanai K, Sakai N, Sakurai E, Maeyama K, Sasaki H and Watanabe T (1995) Effects of thioperamide, a histamine H3 antagonist, on the step-through passive avoidance response and histidine decarboxylase activity in senescence-accelerated mice. Pharmacol. Biochem. Behav. 50: 321-325.
- Monti JM, Jantos H, Leschke C, Elz S and Schunack W (1994) The selective histamine H1-receptor agonist 2-(3-trifluoromethylphenyl)histamine increases waking in the rat. Eur. Neuropsychopharmacol. 4: 459-462.
- Morgan S, Nomikos G and Huston JP (1993) Behavioral analysis of asymmetries induced by unilateral 6-OHDA injections into the substantia nigra. Behav. Neural Biol. 60: 241-250.
- Morgan S, Baker D and Huston JP (1998) Relationship between behavioral recovery from unilateral 6-OHDA lesion of the substantia nigra and changes in the tuberomammillary-striatal projection as measured by HRP labeling. Restor. Neurol. Neurosci. 12: 1-9.
- Nakamura S, Ohnishi K, Nishimura M, Suenaga T, Akiguchi I, Kimura J and Kimura T (1996) Large neurons in the tuberomammillary nucleus in patients with Parkinson's disease and multiple system atrophy. Neurology 46: 1693-1696.
- Olds ME and Olds J (1963) Approach-avoidance analysis of rat diencephalon. J. Comp. Neurol. 120: 259-295.
- Onodera K, Yamatodani A, Watanabe T and Wada H (1994) Neuropharmacology of the histaminergic neuron system in the brain and its relationship with behavioral disorders. Prog. Neurobiol. 42: 685-702.
- Orange PR, Heath PR, Wright SR, Ramchand CN, Kolleiwicz L and Pearson RCA (1996) Allelic variation of the human histamine H2 receptor gene is a major predisposing factor for schizophrenia. Soc. Neurosci. Abstr. 22: 241.
- Panula P, Pirvola U, Auvinen S and Airaksinen MS (1989) Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience 28: 585-610.
- Prell GD, Green JP, Kaufmann CA, Khandelwal JK, Morrishow AM, Kirch DG, Linnoila M and Wyatt RJ (1995) Histamine metabolites in cerebrospinal fluid of patients with chronic schizophrenia: Their relationships to levels of other aminergic transmitters and ratings of symptoms. Schizophr. Res. 14: 93-104.
- Privou C, Knoche A, Hasenöhrl RU and Huston JP (1998) The H1 and H2-blockers chlorpheniramine and ranitidine applied to the nucleus basalis magnocellularis region modulate anxiety and reinforcement related processes. Neuropharmacology (in press).
- Privou C, Li J-S, Hasenöhrl RU and Huston JP (1998) Enhanced learning by post-trial injection of H1- but not H2-histaminergic antagonists into the nucleus basalis magnocellularis region. Neurobiol. Learning Memory (in press).
- Rassnick S and Kornetsky C (1991) L-Histidine attenuates the effects of pentazocine on rewarding brain-stimulation. Life Sci. 48: 1729-1736.
- Ryu JH, Yanai K, Zhao X-L and Watanabe T (1996) The effect of dopamine D1 receptor stimulation on the up-regulation of histamine H3-receptors following destruction of the ascending dopaminergic neurones. Br. J. Pharmacol. 118: 585-592.
- Sannerud CA, Kaminski BJ, Griffiths RR and Roland R (1995) Maintenance of H-sub-1 antagonists self-injection in baboons. Exp. Clin. Psychopharmacol. 3: 26-32.
- Schlicker E, Betz R and Göthert M (1988) Histamine H3 receptor-mediated inhibition of serotonin release in the rat brain cortex. Naunyn-Schmiedebergs Arch. Pharmacol. 337: 588-590.
- Schlicker E, Behling A, Lummen G, Malinowska B and Göthert M (1992) Mutual interaction of histamine H3-receptors and alpha 2-adrenoceptors on noradrenergic terminals in mouse and rat brain cortex. Naunyn-Schmiedebergs Arch. Pharmacol. 345: 639-646.
- Schwarcz R, Hökfelt T, Fuxe K, Jonsson G, Goldstein MD and Terenius L (1979) Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp. Brain Res. 37: 199-216.
- Schwartz JC, Arrang JM, Bouthenet ML, Garbarg M, Pollard H and Ruat M (1991) Histamine receptors in brain. In Histamine and Histamine Antagonists, Uvnäs B, ed. Springer-Verlag, Berlin, pp. 191-242.
- Segura-Torres P, Wagner U, Massanes-Rutger E, Aldavert-Vera L, Marti-Nicolovius M and Morgado-Bernal I (1996) Tuberomammillary nucleus lesion facilitates two-way active avoidance retention in rats. Behav. Brain Res. 82: 113-117.
- Servos P, Barke KE, Hough LB and Vanderwolf CH (1994) Histamine does not play an essential role in electrocortical activation during waking behavior. Brain Res. 636: 98-102.
- Smith CP, Hunter AJ and Bennett GW (1994) Effects of (R)-alpha-methylhistamine and scopolamine on spatial learning in the rat assessed using a water maze. Psychopharmacology 114: 651-656.
- Stäubli U and Huston JP (1979) Up-hill avoidance: A new passive-avoidance task. Physiol. Behav. 22: 775-776.
- Steinbusch HWM and Mulder AH (1984) Immunohistochemical localization of histamine in neurons and mast cells in the rat brain. In Handbook of Chemical Neuroanatomy Vol. 3. Classical Transmitters and Transmitter Receptors in the CNS (Part II), Björklund A, Hökfeld T and Kuhar MJ, eds. Elsevier, Amsterdam, pp. 126-140.
- Steinbusch HWM, Sauren Y, Groenewegen H, Watanabe T and Mulder AH (1986) Histaminergic projections from the premammillary and posterior hypothalamic region to the caudate-putamen complex in the rat. Brain Res. 368: 389-393.
- Steiner H, Bonatz AE, Huston JP and Schwarting R (1988) Lateralized wall-facing versus turning as measures of behavioral asymmetries and recovery of function after injection of 6-hydroxydopamine into the substantia nigra. Exp. Neurol. 99: 556-566.
- Steiner H, Weiler HT, Morgan S and Huston JP (1989) Asymmetries in crossed and uncrossed nigrostriatal projections dependent on duration of unilateral removal of vibrissae in rats. Exp. Brain Res. 77: 421-424.
- Steiner H, Weiler HT, Morgan S and Huston JP (1992) Time-dependent neuroplasticity in mesostriatal projections after unilateral removal of vibrissae in the adult rat: Compartment-specific effects on horseradish peroxidase transport and cell size. Neuroscience 47: 793-806.
- Stutts WA, Orr EL, Lal H and Forster MJ (1996) H3 receptor ligands modulate memory processes in mice. Soc. Neurosci. Abstr. 22: 141.
- Suzuki T, Takamori K, Misawa M and Onodera K (1995) Effects of the histaminergic system on the morphine-induced conditioned place preference in mice. Brain Res. 675: 195-202.
- Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9: 321-353.
- Takada K, Barrett JE, Allen MS, Cook JM and Katz JL (1992) Punishment of schedule-controlled behavior with beta-carboline injections: Antagonism and comparisons with other compounds. J. Pharmacol. Exp. Ther. 261: 138-145.
- Tasaka K, Kamei C, Akahori H and Kitazumi K (1985) The effects of histamine and some related compounds on conditioned avoidance response in rats. Life Sci. 37: 2005-2014.
- Tuomisto L (1994) Regulation of feeding behavior, with special reference to histamine. J. Physiol. Pharmacol. 45: 469-477.
- Unterwald EM, Kucharsky LT, Williams JEG and Kornetsky C (1984) Tripelennamine: Enhancement of brain-stimulation reward. Life Sci. 34: 149-153.
- Vanderwolf CH (1969) Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 26: 407-418.
- Wagner U, Weiler HT and Huston JP (1993) Amplification of rewarding hypothalamic stimulation following a unilateral lesion in the region of the tuberomammillary nucleus. Neuroscience 52: 927-932.
- Wagner U, Segura-Torres P, Weiler T and Huston JP (1993) The tuberomammillary nucleus region as a reinforcement inhibiting substrate: Facilitation of ipsihypothalamic self-stimulation by unilateral ibotenic acid lesions. Brain Res. 613: 269-274.
- Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano Y, Tohyama M and Wada H (1984) Distribution of the histaminergic neuron system in the central nervous system of rats: A fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 295: 13-25.
- Wauquier A and Niemegeers CJE (1981) Effects of chlorpheniramine, pyrilamine and astemizole on intracranial self-stimulation in rats. Eur. J. Pharmacol. 72: 245-248.
- Weiler HT, Steiner H and Huston JP (1990) Plasticity in crossed and uncrossed tuberomammillary-striatal projections in relation to recovery from behavioral asymmetries induced by hemivibrissotomy. Neuroscience 37: 463-469.
- Weiler HT, Wagner U and Huston JP (1992) Unilateral lesion in the tuberomammillary nucleus region: Behavioral asymmetries and effects of histamine precursor. Behav. Brain Res. 49: 167-173.
- Weiler HT, Hasenöhrl RU, Van Landeghem AAL, Van Landeghem M, Brankack J, Huston JP and Haas HL (1998) Differential modulation of hippocampal signal transfer by tuberomammillary nucleus stimulation in freely moving rats dependent on behavioral state. Synapse 28: 294-301.
- Wise RA and Rompre PP (1989) Brain dopamine and reward. Annu. Rev. Psychol. 40: 191-225.
- Yanai K, Watanabe T, Inoue I and Watanabe T (1996) Reduced aggressiveness and impaired exploratory behavior in mice lacking histamine H1 receptors. Soc. Neurosci. Abstr. 22: 2064.
- Yokoyama H, Onodera K, Iinuma K and Watanabe T (1994) 2-Thiazolylethylamine, a selective histamine H1 agonist, decreases seizure susceptibility in mice. Pharmacol. Biochem. Behav. 47: 503-507.
- Yokoyama H, Onodera K, Maeyama K, Sakurai E, Iinuma K, Leurs R, Timmerman H and Watanabe T (1994) Clobenpropit (VUF-9153), a new histamine H3 receptor antagonist, inhibits electrically induced convulsions in mice. Eur. J. Pharmacol. 260: 23-28.
- Zimmermann P, Wagner U, Krauth J and Huston JP (1997) Unilateral lesion of dorsal hippocampus enhances reinforcing lateral hypothalamic stimulation in the contralateral hemisphere. Brain Res. Bull. 44: 265-271.
| Discussion Board | Previous Page | Your Symposium |