Invited Symposium: Neuronal Histamine Systems and Behavior
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
There is agreement now that histamine and histaminergic systems have an important role in several physiological processes in the brain (Onodera et al., 1994; Yokoyama & Iinuma, 1996; Alvarez & Banzan, 1995, 1996a,b; Ruarte et al., 1997). Our laboratory has been interested for many years to characterize the possible functional role of histamine and its receptors in some specific brain processes such as cognition and exploratory motivation. Evidence has been found suggesting that some structures of the limbic system and related neural components appear to be involved in these mechanisms (Grossberg & Merrill, 1996; Eichenbaum, 1996; Alvarez & Banzan, 1995, 1996a,b; Salamone, 1992; West et al., 1992; Maldonado et al., 1995; Orofino & Alvarez, 1996a,b). Since histamine receptors have been located in the hippocampus and also in the nucleus accumbens ( Steinbusch et al., 1986; Inagaki et al., 1988) it was of interest to examine these structures in connection to cognitive and motivation processes in the rat.
Materials and Methods
Two experimental approaches were used: (i) learning of an active avoidance response, as a model of memory processes, and (ii) exploration in a conflictive environment, as a model of motivation-emotionality processes.
Learning of an active avoidance response.
The one-way active avoidance response to an ultrasonic tone of 40 kHz as outlined in Figure 1, was used.
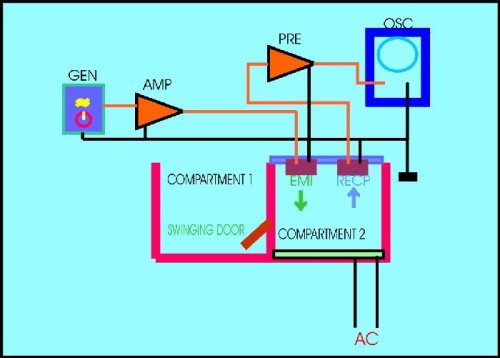 Fig.1 Experimental design of the ultrasonic apparatus used as a model of learning of an active avoidance response.
AMP= Power ultrasonic amplifier. EMI= ultrasonic emitter. GEN= ultrasonic sinewave generator. OSC= osciloscope. PRE= ultrasonic preamplifier. RECP= ultrasonic receptor.
Fig.1 Experimental design of the ultrasonic apparatus used as a model of learning of an active avoidance response.
AMP= Power ultrasonic amplifier. EMI= ultrasonic emitter. GEN= ultrasonic sinewave generator. OSC= osciloscope. PRE= ultrasonic preamplifier. RECP= ultrasonic receptor.
During the adaptation period prior to the training trials, animals are allowed to pass from compartment 2 (the punishment box) to compartment 1 (the safe box) through the swinging door that can be locked in its place. Rats were conditioned to escape through by releasing the block of the door after the ultrasonic sinewave tone was on. When animals avoided the electric shock passing through the door before the ultrasonic tone was off, a "positive" response was considered. If the animal failed to escape after 60 sec the ultrasonic tone was off, a "negative" response was considered. Electric shocks were given at a rate of 1 each 15 sec. The total duration of the ultrasonic tone was 30 sec, so rats have up to 30 sec to avoid the punishment. The time the animals take after the onset of the conditioning tone to avoid the electric shock was the "latency time". The sum of the positive responses during a session, divided by the number of trials was the "percentage of conditioned avoidance responses (% of CAR)". A total of 8 trials compose one session and two sessions were necessary for control animals to learn the avoidance task. Additional details have been described elsewhere (Alvarez & Banzan, 1995, 1996a).
Exploration in a conflictive environment.
The elevated asymmetric plus-maze (APM) was used as a model of a novel conflictive environment for studying exploratory motivation in rats. General outline of the APM is shown in Figure 2.
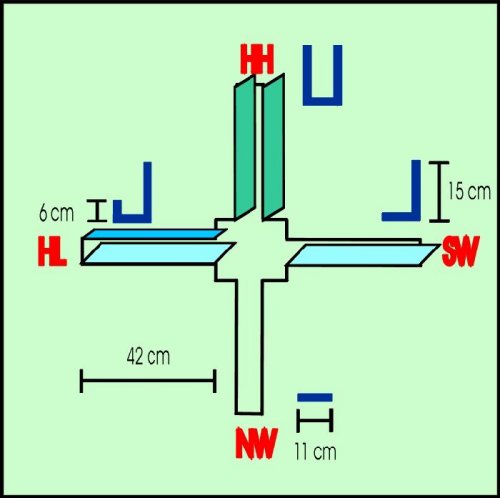 Fig. 2. Simplified diagram of the APM. NW= no wall, SW= a single wall. HL= a high and a low wall. HH= two high walls. Rats were placed one at a time in the center platform at the onset of the test. Behavioral activity displayed was measured by the automatic increment of a digital counting device operated by an observer.
Fig. 2. Simplified diagram of the APM. NW= no wall, SW= a single wall. HL= a high and a low wall. HH= two high walls. Rats were placed one at a time in the center platform at the onset of the test. Behavioral activity displayed was measured by the automatic increment of a digital counting device operated by an observer.
The degree of asymmetry of each arm was as follows: no wall (NW), ii) single wall (SW, 15 cm high), iii) two walls (HL, one of them 15 cm high and the other 6 cm high), and iv) two high walls (HH, both 15 cm high). The APM was elevated 60 cm from the floor and placed in the center of the experimental room, which was illuminated by a single 30-W fluorescent lamp above the APM. Rats were tested in the APM only once. At the onset of the test, rats were individually placed in the center platform and during 5 min all behavioral activity displayed was videotaped. At a later time, behaviors were measured by an observer operating a digital counting device which displayed counts at a rate of about 2 counts/sec. The following variables were measured: i) exploration score: the exploratory activity displayed by the animal in any arm. Included in this category were the following behaviors: i.1.) Locomotion into any arm, sniffing to any side while the rat walks in or out the arm; i.2) rearing on any wall or in the air during at least 2 sec; i.3) sniffing a localized spot in any arm for at least 2 sec, and i.4) head-dipping at the end of any arm for at least 2 sec. The exploration score was considered an index of exploratory motivation. ii) Permanency score (stationary behavior): all the non-exploratory activity displayed in any arm while the rat is stationary in some point. Grooming, rest immobilization for at least 2 sec and sleeping were the behaviors included in this category. Since these behaviors are usually displayed by the animal while is in a non-stressful place, the permanency score was considered an inverse gross index of emotionality (Ruarte et al., 1997).
General procedures.
Animals were implanted with guide cannulas for microinjection into the ventral hippocampus or into the medial nucleus accumbens. All rats were resting for 72 h before any experimental treatment was applied. After this recovery period, rats implanted into the hippocampus were subjected to the learning schedule, and rats implanted into the medial nucleus accumbens were subjected to the 5 min sessions in the APM of the motivation-emotionality schedule. Once the experiments were over, all animals were sacrificed with ether excess and their brains dissected for histological verification of sites of implant. Sites of microinjections for the hippocampus were restricted to the ventral zone, including the CA2-CA4 region and were located in a coronary plane at about 3.2 mm caudal to bregma, referring to the Pellegrino's rat brain co-ordinates atlas (1979). For the nucleus accumbens, microinjections were restricted to the most ventral zone in a coronary plane at about 3.2 mm rostral to bregma, referring to the Pellegrino's rat brain co-ordinates atlas (1979). Results are expressed as the median ± the standard error of the median.
Drugs used. Histamine dihydrochloride (HA) and pyrilamine maleate (PYR) as H1-histamine antagonist, or ranitidine (RAN) as H2-histamine antagonist were used. A relation of antagonist to agonist of 1:1 or 1.5:1 was used. The following protocol for drugs administration was used for all type of experiments (Figure 3):
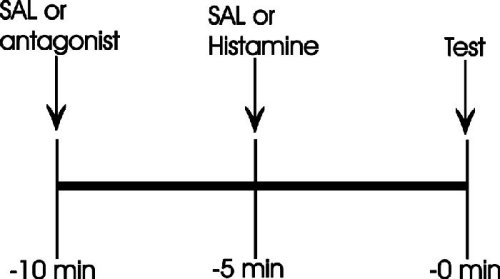 Fig. 3. Experimental protocol for drugs administration.
Fig. 3. Experimental protocol for drugs administration.
Results
As shown in Figure 4, the localized application of histamine into the ventral hippocampus increased significantly the latency time to show the avoidance response. Control rats typically show a median of about 8 sec, while histamine-treated rats were over the 30 sec limit for 6 consecutive trials (Figure 4).
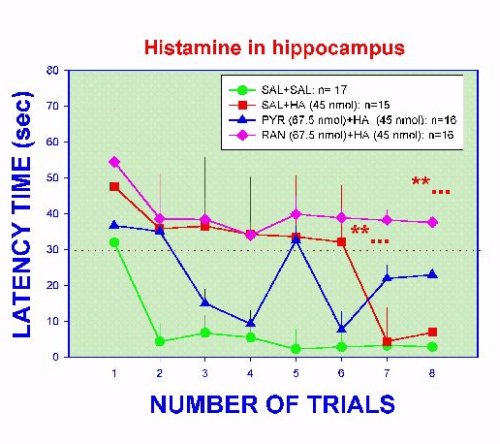 Fig. 4. Latency time to show an avoidance response to an ultrasonic tone in implanted rats microinjected with histamine and/or histamine antagonists. Dashes line indicate the 30 sec limit to show the response. **...= p <0.01 versus SAL curve, except trial 1 in the RAN group and trials 7 and 8 in HA group.
Fig. 4. Latency time to show an avoidance response to an ultrasonic tone in implanted rats microinjected with histamine and/or histamine antagonists. Dashes line indicate the 30 sec limit to show the response. **...= p <0.01 versus SAL curve, except trial 1 in the RAN group and trials 7 and 8 in HA group.
Eventually, histamine-treated rats had a latency time not different from control animals. These data show that histamine is inhibitory to the learning process. It is known that histamine interferes with the evocation of the learned avoidance response (Alvarez & Banzan, 1995), so present results are not surprising since a deleterious recall mechanism should affect the process of learning. The inhibitory effect of histamine is blocked when pyrilamine is administered at the same time that histamine (Figure 4). Interestingly, the application of ranitidine in the same conditions did not abolish the effect of histamine. On the contrary, the inhibitory action of histamine is increased (Figure 4). Considering that the latency time is related to the hippocampal neuronal mechanisms participating in the "processing" of the input stimuli, this evidence suggest that H1-histamine receptors are apparently involved in these mechanisms. Data from Figure 3, also suggest that H2-histamine receptors seem to facilitate in some way the normal recall phase, perhaps modulating the inhibitory action of the H1-histamine receptors. Analyzing the efficiency of learning, which is reflected by the % of CAR during the training sessions, a more clarifying picture is obtained (Figure 5).
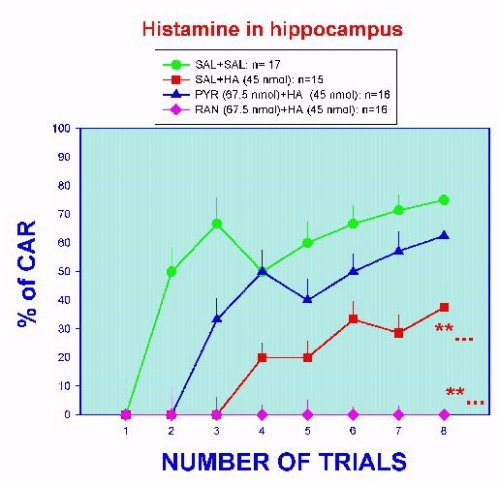 Fig. 5. Learning efficiency in hippocampal implanted rats treated with histamine and/or histamine antagonists. **...= p < 0.01 versus SAL curve, except trial 1.
Fig. 5. Learning efficiency in hippocampal implanted rats treated with histamine and/or histamine antagonists. **...= p < 0.01 versus SAL curve, except trial 1.
Histamine treatment significantly diminish the percentage of accumulated positive responses in time. The histamine curve is displaced to the right and at the end of the session a low score is reached (about 40 %). As shown in the figure, only the pyrilamine treatment was able to counteract the deleterious effect of histamine. Learning efficiency under the simultaneous treatment of ranitidine and histamine was completely abolished. Data strongly suggest the participation of H1- and H2- histamine receptors in memory processes in the hippocampus.
The second point of interest in our laboratory was centered in the motivational mechanisms of the brain. Although the term "motivation" has been restricted usually to those brain processes that recognize sex, food or thirst as primary stimuli, there is evidence that the brain is able to incorporate some other sensorial stimuli also (Montgomery, 1954; Berlyne, 1967; Robbins & Everitt, 1996; Hunt et al., 1994; Beninger & Miller, 1998; Ruarte et al., 1997).
In a wider context, motivation is viewed as a brain mechanism that process incentive stimuli originated in the environment in such a way that behavioral patterns of approach, rejection or indifference to the stimuli are produced if the "environmental clues" are motivating or non-motivating. However, it is very simplistic to consider that only this aspect control exploration of a novel environment. When some characteristic in the environment represents some risk to the animal, the so called "conflictive environment", fear plays an important role in the final behavioral pattern showed by the animal.
We have considered the APM as an appropriate experimental approach to a conflictive environment. Since maze arms are asymmetric in geometrical characteristics, the apparatus gives to rats a graduated motivating (or fearful) stimuli spectrum (Figure 2). Restricting observations to only those behavioral patterns related to exploration and to emotionality (fear) a better physiological characterization of brain mechanisms has been assumed. It is interesting to consider that the nucleus accumbens have been implicated in motivational processes (Salamone, 1992; Stern & Passingham, 1991; Apicella et al., 1991; Wise, 1996; Robbins & Everitt, 1996; Schwarting et al., 1998; Berns et al., 1997; Orofino & Alvarez, 1996a,b). Behavioral expression in the APM after chemical stimulation of the accumbens nucleus with histamine is shown in Figures 6 and 7.
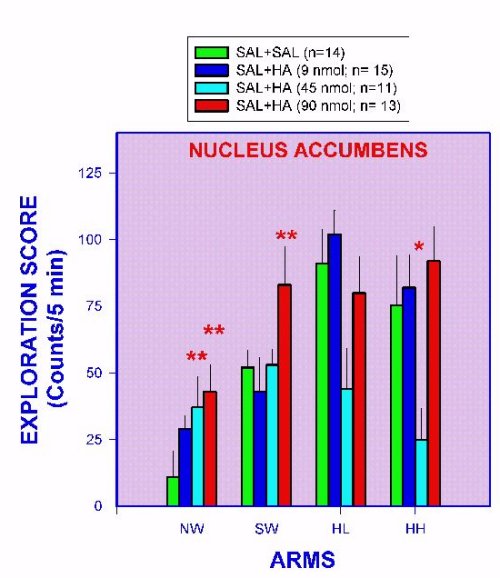 Fig. 6. Exploratory behavior as measured in the APM of nucleus accumbens-implanted rats injected with histamine. **= p < 0.01 versus SAL+SAL group.
Fig. 6. Exploratory behavior as measured in the APM of nucleus accumbens-implanted rats injected with histamine. **= p < 0.01 versus SAL+SAL group.
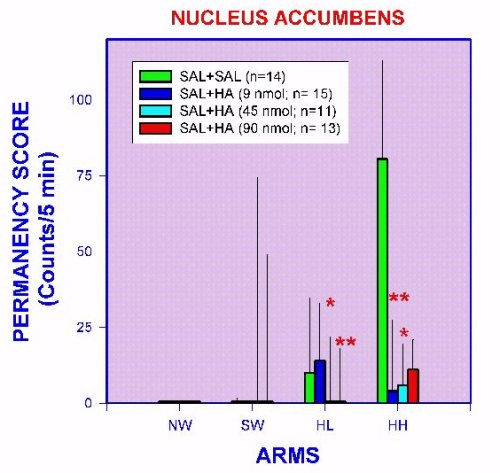 Fig. 7. Non-exploratory stationary behaviors (permanency), measured in the APM of nucleus accumbens-implanted rats injected with histamine. *= p < 0.05 versus SAL+SAL group. **= p < 0.01 versus SAL+SAL group.
Fig. 7. Non-exploratory stationary behaviors (permanency), measured in the APM of nucleus accumbens-implanted rats injected with histamine. *= p < 0.05 versus SAL+SAL group. **= p < 0.01 versus SAL+SAL group.
Control rats show the higher exploration scores in the HL and HH arms, significantly less in the HL and even less in the NW arm (Figure 6). Non-exploratory behaviors related to emotionality (permanency) are present only in the HL and HH arms (Figure 7). These results suggest that NW and SW are viewed as highly fear-inducing clues but at the same time as attracting stimuli to control rats, since in spite of fear, exploration is performed anyway. Under this context it is possible to speculate that exploration in the fear-inducing arms, is a reflection of exploratory motivation processes. Intra-accumbens histamine injection induced a clear increase in exploration in the SW and NW arms with no changes in the permanency score (Figures 6 and 7). Accordingly, it can be inferred that histamine neurons in the nucleus accumbens are facilitating the motivation mechanisms. It is interesting to point out that the histamine effect in the less fear-inducing arms of the APM (HL and HH arms) is a decrease of permanency (Figure 7). This finding can be interpreted as histamine neurons in the nucleus accumbens can modulate the fear-inducing "content" of environmental clues. These effects of histamine in accumbens neurons seem in part to be mediated by H1- and H2- histamine receptors, since a blocking of the histamine effects is produced by intra-accumbens pre-treatment with pyrilamine and ranitidine (Figures 8 and 9).
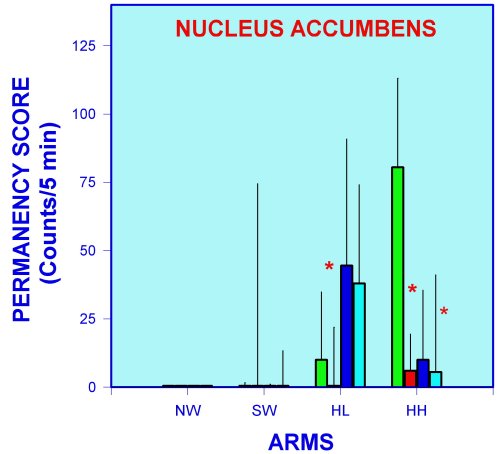 Fig. 8. Exploration behavior as measured in the APM of nucleus accumbens-implanted rats injected with histamine and/or histamine antagonists. **= p < 0.01 versus SAL+SAL group. Groups (SAL) and (HA) reproduced from figure 6 to facilitate comparisons.
Fig. 8. Exploration behavior as measured in the APM of nucleus accumbens-implanted rats injected with histamine and/or histamine antagonists. **= p < 0.01 versus SAL+SAL group. Groups (SAL) and (HA) reproduced from figure 6 to facilitate comparisons.
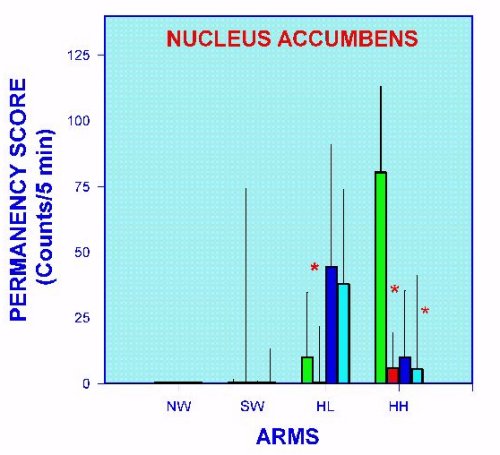 Fig. 9. Non-exploratory stationary behaviors measured in the APM, of nucleus accumbens-implanted rats injected with histamine and/or histamine antagonists. *= p < 0.05 versus SAL+SAL group.
Fig. 9. Non-exploratory stationary behaviors measured in the APM, of nucleus accumbens-implanted rats injected with histamine and/or histamine antagonists. *= p < 0.05 versus SAL+SAL group.
It is interesting to mention that some effects attributed to histamine seem not to be related to H1- or H2- histamine receptors (Figure 9). Considering that only one dose of histamine antagonists was used, it is not possible for the moment to exclude the participation of these receptors on the histamine effects.
Discussion and Conclusion
As concluding remarks, present evidence offers an additional support to the concept that histamine and its receptors in neurons of the limbic and related structures, play a significant role in specific physiological mechanisms of the brain, such as cognition and motivation-emotionality processes. As always happen in this type of studies, some questions are completely answered, others are poorly solved and new ones are raised. Our laboratory continues the research to clarify the poorly answers and to solve the new ones.
Acknowledgments
This study has been supported by grants from FONCyT (Agencia Nacional de Promoción Científica y Tecnológica, proyecto PICT 0007) and SECyT-U.N.Cuyo (Proyecto 06/J039).
References
- Alvarez EO, Banzan AM (1995) Effects of localized histamine microinjection into the hippocampal formation on the retrieval of a one-way active avoidance response in rats. The Journal of Neural Transmission 101: 201-211
- Alvarez EO, Banzan AM (1996a) Hippocampal histamine receptors: possible role on the mechanisms of memory in the rat, II. The Journal of Neural Transmission 103: 147-156
- Alvarez EO, Banzan AM (1996b) Hippocampus and learning. Possible role of histamine receptors. Medicina 56: 155-160
- Apicella P, Ljundberg T, Scarnati E, Schultz W (1991) Responses to reward in monkey dorsal and ventral striatum. Experimental Brain Research 85: 491-500
- Beninger RJ, Miller R (1998) Dopamine D1-like receptors and reward-related incentive learning. Neuroscience and Biobehavioral Reviews 22: 335-345
- Berlyne DE (1967) Arousal and reinforcement. In Levine D (editor) Nebraska Symposium on Motivation. University of Nebraska Press, Lincoln, Nebraska, pp 1-110
- Berns GS, Cohen JD, Mintun MA (1997) Brain regions responsive to novelty in the absence of awareness. Science 276: 1272-1275
- Eichbaum H (1996) Is the rodent hippocampus just for "place"? Current Opinion in Neurobiology 6: 187-195
- Grossberg S, Merrill JWL (1996) The hippocampus and cerebellum in adaptively timed learning, recognition, and movement. Journal of Cognitive Neuroscience 8: 257-277
- Hunt GE, Atrens DM, Jackson DM (1994) Reward summation and the effects of dopamine D-1 and D-2 agonists and antagonists on fixed-interval responding for brain stimulation. Pharmacology, Biochemistry and Behavior 48: 853-862
- Inagaki N, Yamatodani A, Ando-Yamamoto M, Tohyama M, Waatanabe T, Wada H (1988) Organization of the histaminergic fibers in the rat brain. The Journal of Comparative Neurology 273: 283-300
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. The Journal of Neuroscience 15: 6779-6788
- Montgomery KC (1954) The role of the exploratory drive in learning. Journal of Comparative Physiology and Psychology 44: 582-589
- Onodera K, Yamatodani A, Watanabe T, Wada H (1994) Neuropharmacology of the histaminergic neuron system in the brain and its relationship with behavioral disorders. Progress in Neurobiology 42: 685-702
- Orofino AG, Alvarez EO (1996a) Papel del ácido glutámico y la histamina en el núcleo accumbens sobre la motivación y la emocionalidad en la rata. Acta Physiologica Pharmacologica et Therapeutica Latinoamericana 46: (Suppl 1), 58 (abstract)
- Orofino AG, Alvarez EO (1996b) Respuestas conductuales por estimulación con histamina en el núcleo accumbens de ratas adultas: motivación y emocionalidad. Acta Physiologica Pharmacologica et Therapeutica Latinoamericana 46: (Suppl 1), 58 (abstract)
- Pellegrino LJ (1979) A stereotaxic atlas of the rat brain. Plenum Press, New York
- Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology 6: 228-236
- Ruarte MB, Orofino AG, Alvarez EO (1997) Hippocampal histamine receptors and conflictive exploration in the rat: studies using the elevated asymmetric plus-maze. Brazilian Journal of Medical and Biological Research 30: 1451-1461
- Salamone JD (1992) Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology 107: 160-174
- Schwarting RKW, Thiel CM, Müller CP, Huston JP (1998) Relationship between anxiety and serotonin in the ventral striatum. Neuroreport 9: 1025-1029
- Steinbush HWM, Sauren H, Groenwegen H, Watanabe T, Mulder AH (1986) Histaminergic projections from premammillary and posterior hypothalamic region to the caudate-putamen complex in the rat brain. Brain Research 368: 368-393
- Stern CE, Passingham RE (1991) The nucleus accumbens in monkeys (Macaca fascicularis): incentive and emotion. Society of Neuroscience 17: 664 (abstract)
- West HK, Clancy AN, Michael RP (1992) Enhanced responses of nucleus accumbens neurons in male rats to novel odors associated with sexually receptive females. Brain Research 585: 49-55
- Wise RO (1996) Neurobiology of addiction. Current Opinion in Neurobiology 6: 243-251
- Yokoyama H, Iinuma K (1996) Histamine and seizures. Implications for the treatment of epilepsy. CNS Drugs 5: 321-330
| Discussion Board | Previous Page | Your Symposium |