Invited Symposium: Molecular Mechanisms of Ageing
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Recently, we and other groups proposed a nucleation-dependent polymerization model to explain the mechanisms of Alzheimer's ß-amyloid fibril (fAß) formation in vitro (1, 2). We have developed a first order kinetic model of fAß extension in vitro and confirmed that the extension of fAß proceeds via the consecutive association of amyloid ß-peptides (Aß) onto the ends of existing fibrils (2). However, no convincing kinetic models to explain the characteristic sigmoidal time-course curve of fAß formation from Aß at a physiological pH, have been reported. Although many research groups have extensively examined the effect of apoE on fAß formation in vitro, the results are somewhat conflicting (3, 4). On the other hand, several findings strongly suggest that free radicals play an important role in the fAß formation process (5). Here, we establish a kinetic model to explain the characteristic sigmoidal time-course curve of fAß formation from Aß. Based on this model, we then compare the mechanisms of apoE- and AO-mediated inhibition of fAß formation in vitro.
Materials and Methods
The reaction mixture contained 50 mM phosphate buffer, pH 7.5, 100 mM NaCl, 5-30 µM Aß(1-42), or 10-50 µM Aß(1-40), and 0-100 nM of recombinant human apoE3 or AO (0-100 µM of rifampicin (RIF), 0-30 µM of nordihydroguaiaretic acid (NDGA)). The reaction was initiated by shifting the temperature to 37 °C, with a DNA thermal cycler. At each incubation time, the reaction of the corresponding tube was stopped by placing the tubes on ice and analyzed by fluorescence spectroscopy with thioflavin T (ThT) (2).
Results
Kinetics of fAß formation from fresh Aß. As shown in Figure 1A, when fresh Aß(1-42) was incubated at 37 °C, the fluorescence of ThT (F(t)) followed a characteristic sigmoidal curve. Plotting the fluorescence data as common logarithms, shown in Figure 1B, gives a linear semilogarithmical plot. Interpretation of these plots yields the following differential equation: F'(t)=BF(t)(A-F(t)) (1) where A is tentatively determined as F(infinity), B is a constant, and F'(t) represents the rate of fluorescence increase at a given time. Eq 1 is a logistic differential equation and clearly shows that the above-described sigmoidal curve is a logistic curve. When F(t)=A/2, F(t)/A-F(t)=1. Moreover, from eq 1, F'(t) reaches its maximum. Therefore, from the straight line shown in Figure 1B, we obtain the time when F'(t) is maximum. We describe this time point as t1/2. Similar sigmoidal curves and semilogarithmical plots were obtained in the case of fresh Aß(1-40).
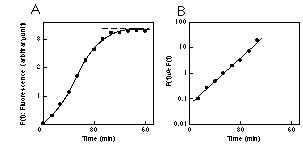 Fig. 1: Kinetics of fAß(1-42) formation from fresh Aß(1-42). A: Time course of the fluorescence after the initiation of the reaction. B: The semilogarithmical plots of the value: F(t)/A-F(t) versus incubation time. A is tentatively determined as F(infinity) and shown as a broken line on a sigmoidal curve.
Fig. 1: Kinetics of fAß(1-42) formation from fresh Aß(1-42). A: Time course of the fluorescence after the initiation of the reaction. B: The semilogarithmical plots of the value: F(t)/A-F(t) versus incubation time. A is tentatively determined as F(infinity) and shown as a broken line on a sigmoidal curve.
Effects of apoE and AO on the kinetics of fAß formation from fresh Aß. Although apoE extended t1/2 of both Aß(1-42) and (1-40) in a dose dependent manner, AO did not. On the other hand, the final amount of fAß formed was decreased by both apoE and AO dose-dependently. Effects of apoE and AO on the kinetics of fAß extension. We then analyzed the effect of apoE and AO on the extension reaction of fAß, based on a first-order kinetic model (2, 4). Although apoE extended the time to proceed to equilibrium in a dose-dependent manner, AO did not. On the other hand, both apoE and AO dose-dependently decreased the final amount of fAß formed.
Discussion and Conclusion
ApoE may inhibit the extension of fAß in vitro and extend the time to proceed to equilibrium, by making a complex with Aß, thus eliminating free Aß from the reaction mixture (4). Thus, we hypothesized that apoE would inhibit the nucleus formation by making a complex with Aß and/or prenuclei. Actually, apoE3 extended t1/2 in a dose-dependent manner (see above). In sharp contrast to apoE, AO had no effect on t1/2 or the time to proceed to equilibrium in the extension reaction (see above). Therefore, it is not likely that AO would inhibit fAß formation by making a complex with Aß and/or prenuclei. Further studies are necessary to clarify the mechanisms by which AO inhibit fAß formation in vitro. Comparison of the inhibitory effects of apoE and AO clearly indicates two pharmacological strategies that could be applied to inhibit or retard fAß deposition in vivo.
References
- Jarrett, JT and Lansbury, PT Jr (1993) Seeding "One-dimensional crystallization" of amyloid: A pathogenic mechanism in Alzheimer's disease and scrapie? Cell, 73: 1055-8.
- Naiki, H. and Nakakuki, K (1996) First-order kinetic model of Alzheimer's ß-amyloid fibril extension in vitro. Lab Invest, 74: 374-83.
- Wisniewski, T, Castano, EM, Golabek, A, Vogel, T and Frangione, B (1994) Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol, 145; 1030-5.
- Naiki, H, Gejyo, F and Nakakuki, K (1997) Concentration-dependent inhibitory effects of apolipoprotein E on Alzheimer's ß-amyloid fibril formation in vitro. Biochemistry, 36: 6243-50.
- Markesbery, WR (1997) Oxidative stress hypothesis in Alzheimer's disaese. Free Radic Biol Med, 23: 134-47.
| Discussion Board | Previous Page | Your Symposium |