Invited Symposium: Na-H Exchangers and Intracellular pH Regulation
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
INTRODUCTION
pH changes in nerve and glial cells and in the extracellular space in nervous systems can be evoked by neuronal activity, by neurotransmitters, by active cellular pH regulation and by metabolic processes. Rapid pH transients may actually be signals rather than only a result of inadequate homeostatic acid-base regulation. Analogous to the signalling pattern of other ions, such as e.g. calcium and potassium, transient shifts of protons and bicarbonate, together with the gas carbon dioxide, may influence or initiate functional processes in the nervous system, including pH-induced changes of neuronal excitability, the modulation of gap junctions and thus of electrical synapses and the glial syncytium, and the control of enzyme activities. Proton signalling in cells and in local extracellular domains, as e.g. in the vicinity of synapses, could well contribute to information processing in nervous systems (Deitmer and Rose 1996), and to slow potential shifts associated with neuronal activity.
PH SIGNALLING INDUCED BY NEURONAL ACTIV
Studies on central nervous systems of both vertebrates and invertebrates have shown that neuronal activity leads to defined extra- and intracellular pH changes (Deitmer and Rose 1996). These consist of mono- or multiphasic pH shifts indicating that they might originate from multiple sources and/or via multiple pathways. Due to the increase in extracellular potassium activity, glial cells respond to the activity of neighbouring neurons by a depolarization of their cell membrane.
In the cortex, the stimulus-evoked glial depolarization was accompanied by an intracellular alkalinization of astrocytes, the amplitude of which was dependent on the amplitude of the glial depolarization (Chesler and Kraig 1989).
Several lines of evidence suggest that the depolarization-induced alkalinization of glial cells in both vertebrate and invertebrate preparations is due to inward transport of bicarbonate via an electrogenic Na+/HCO3--cotransport activated by the K+-induced membrane depolarization (Deitmer and Szatkowski 1990; Grichtenko and Chesler 1994; Bevensee et al., 1997).
In the cortex, the glial alkaline shift was partly inhibited in Na+-free saline and turned into a small acidification during the application of Ba2+ (Chesler and Kraig 1989, Grichtenko and Chesler 1994). The stimulus-induced alkalinization of glial cells in the leech was turned into an acidification by all experimental protocols preventing the activation of Na+/HCO3--cotransport: (1) by voltage-clamping the glial cell (Fig. 1 A; Rose and Deitmer 1994), (2) in the presence of the stilbene DIDS and (3) in CO2/HCO3--free saline (Rose and Deitmer 1995 a, b). Suppressing the glial depolarization during nerve root stimulation did not only reverse the intraglial pH change, but also influenced the pHe transient (Fig. 1 B; Rose and Deitmer 1994).
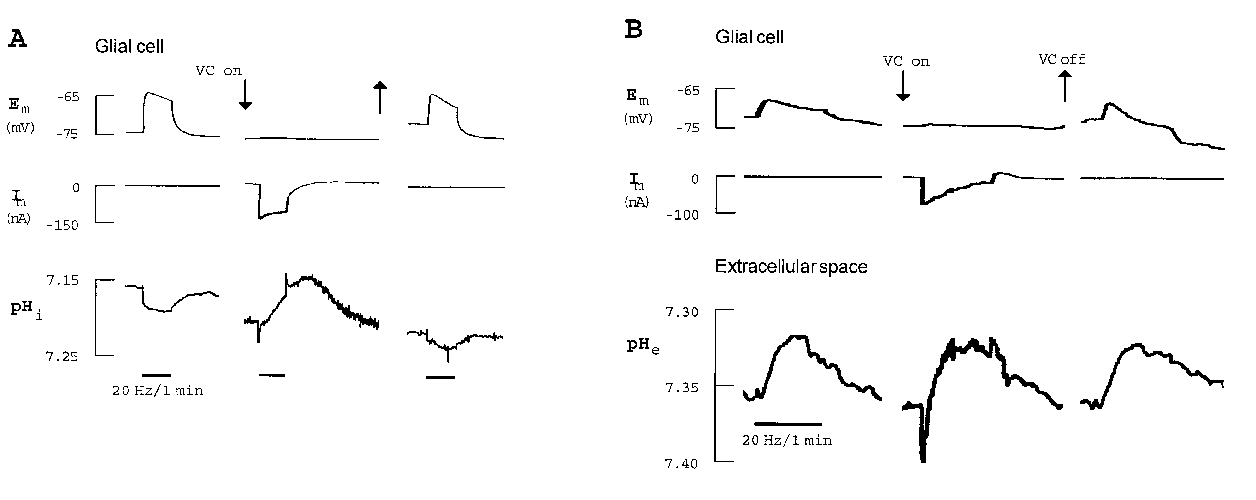 click to enlarge
click to enlarge
Fig. 1.
Glial cells have a variety of transmitter receptors coupled to ion channels (Hösli and Hösli 1993). The activation of these transmitter receptors can induce pH transients in nervous systems, which either emerge directly in response to the action of neurotransmitters themselves by both HCO3--dependent and -independent mechanisms, or are secondary to membrane potential changes (Chesler and Kaila 1992; Deitmer and Munsch 1994). The majority of available data is on GABA- and glutamate-induced pH changes; while the effects of other transmitters, like acetylcholine or serotonin, on extra- or intracellular pH in the nervous system have been investigated in only a few reports. It has become clear, however, that there may be complex interactions between transmitter effects on both glial cells and neurons and extra- and intracellular pH changes, respectively.
pH REGULATION
Like many other cell types, glial cells seem to use transport systems for their pHi regulation. They share with most cells the use of a Na+/H+ exchanger and a Cl-/HCO3- exchanger; with most epithelial cells they share a Na+-HCO3- cotransporter, which is absent in neurons and most non-epithelial cells. The steady-state pHi and the pHi changes in leech glial cells were unaffected by intracellular Ca2+ transients evoked by membrane depolarization (Deitmer, Schneider and Munsch 1993), suggesting that the homeostasis of H+ and Ca2+ is not interrelated in these glial cells. In contrast, an intracellular alkalinization has recently reported in rat astrocytes following activation of metabotropic glutamate receptors, which appeared to be related to intracellular Ca2+ transients (Amos & Chesler, 1998).
In most of these studies, the Na+-HCO3- cotransporter was detected during addition or removal of CO2/HCO3-. Addition of CO2/HCO3- resulted in an intracellular alkaline shift and a rise in intracellular Na+ (Na+i), while removal of CO2/HCO3- reversed these pHi and Na+i changes (Fig. 2 A). Simultaneously, the glial membrane hyperpolarized and depolarized, respectively, during these buffer changes due to reversibility of this electrogenic cotransporter (Deitmer 1991). When voltage-clamped an outward and inward current, respectively, was recorded (Munsch and Deitmer 1994). As in epithelial cells, the Na+-HCO3- cotransporter in glial cells could be inhibited by stilbene derivatives, such as DIDS, SITS or DNDS, but was unaffected by amiloride. In cultured rat astrocytes DIDS was shown partly to block pHi recovery and the CO2/HCO3--dependent outward current (Brune et al 1994) as has also been shown in leech glial cells (Deitmer and Schlue 1989; Deitmer 1991).
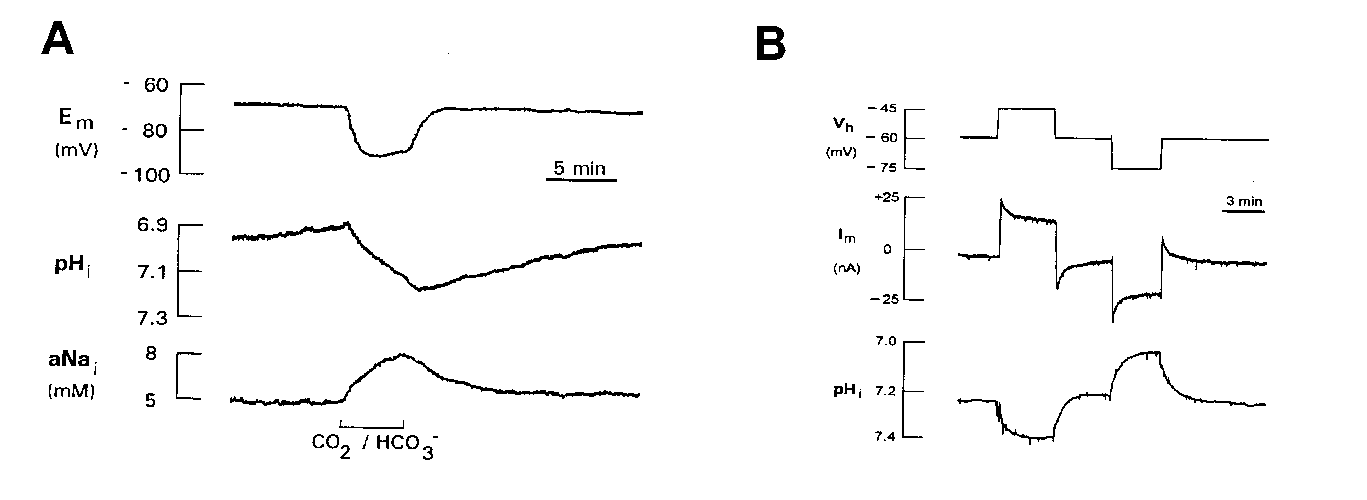 click to enlarge
click to enlarge
Fig. 2.
These CO2/HCO3--dependent pHi shifts and membrane potential changes were dependent upon the presence of external Na+, but not on external or intracellular Cl- concentrations. There was a reversible rise in Na+i during the exposure to CO2/HCO3- in leech glial cells (Fig. 2 A; Deitmer 1992) and cultured rat hippocampal astrocytes (Rose and Ransom 1996).
Reduction of the external pH by reducing the HCO3- concentration evoked an inward current in leech glial cells (Deitmer 1991; Munsch and Deitmer 1994) and rat astrocytes (Brune et al 1994). This is consistent with activation of an outward-going electrogenic Na+-HCO3- cotransport. The cotransporter could also be reversed in retinal Müller glial cells (Newman 1991), i.e. operating inwardly and outwardly, depending on the thermodynamic conditions. This is also reflected by the pHi changes induced by slow voltage steps in voltage-clamped leech neuropile glial cells (Fig. 2 B). De- and hyperpolarizing voltage steps resulted in an intraglial alkalinization and acidification, respectively, providing an extrapolated change of one pH unit/110 mV membrane potential change, indicating a stoichiometry of 2 HCO3- : 1 Na+ (Deitmer and Schneider 1995). The cotransporter appears to a have remarkable high affinity for HCO3-, since it was found active even in the nominal absence of CO2/HCO3- (Deitmer & Schneider, 1998).
PH SHIFTS IN THE NEURON-GLIA DIALOGUE
pH pertubations can induce a variety of changes in cellular functions in nervous systems, from the induction or inhibition of ionic currents, to alterations in the overall neuronal excitability, and to modulation of enzyme activities (Chesler 1990; Deitmer and Rose 1996). In glial cells in particular, pH shifts may be associated with acid/base secretion, lactate transport, cell volume changes, glutamate uptake, alteration in gap junctional communication and metabolic processes. Some of these pH-dependent processes are linked to or might induce, a cascade of H+-induced signals in nervous systems. pH shifts and brief pH transients may contribute to shape neuronal performance, and hence behaviour, at molecular and cellular levels.
Protons are diverse modulators and mediators of many neural functions. They can be regarded as signalling molecules themselves. Glial cells possess an array of mechanisms (Fig. 3) which regulate and change intraglial pH as well as the pH in the ECS. Most of these pH transients are anticipated to be very discrete transients, i.e. very brief and local pH changes, under normal physiological conditions. They may also be considerably larger in amplitude in vivo as compared to those recorded with invasive recording techniques. The largest, and probably most significant pHi changes appear to be contributed by the neurotransmitters, by Na+/H+ exchange and by the reversible, electrogenic Na+-HCO3- cotransporter as well as by organic acid transporters and metabolic processes. With respect to the action of neurotransmitters on pHi and most pHi regulating mechanisms, glial cells appear to resemble neurons, while the electrogenic Na+-HCO3- cotransporter, reminiscent of epithelial cells, exists exclusively in glial cells in nervous systems.
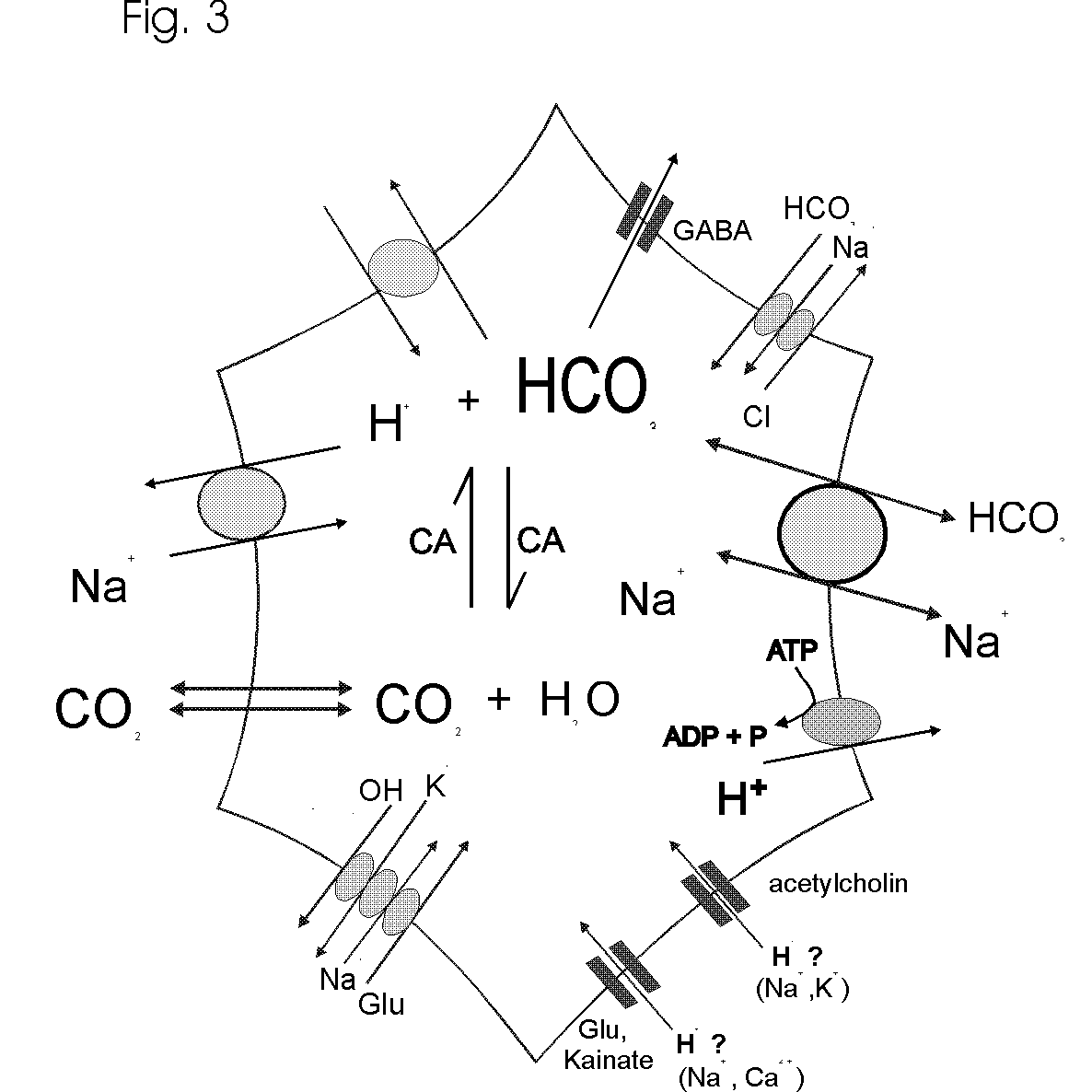 click to enlarge
click to enlarge
Fig. 3.
The action of neuronal electrical activity, neurotransmitters and the H+/HCO3--transporting carriers in the cell membranes produce pH shifts in neurons, ECS and glial cells. It seems often necessary to monitor pH in all three compartments in order to understand the mechanisms of the acid and alkaline transients. Often, the pH shifts in the ECS are mirror images of intracellular pH changes; however, different kinetics and spatial extend of intra- and extracellular pH transients can make it difficult to match e.g. intracellular acidosis with extracellular alkalosis. In addition, we still know little about intracellular processing of H+ about the significance, capacity and time course of organellar H+ sequestration and storage. Although we have long accepted that most biological processes display some pH dependence, we know much less about the involvement of H+ than about e.g. the role of Ca2+, in initiating and shaping signalling cascades in and between cells. For nearly all events related to pH changes in nervous tissue, the presence of CO2 and HCO3- has a great impact. HCO3- determines the buffering power to a large extent, it enables HCO3- flux through GABAA receptor channels and thereby modifies the inhibitory synaptic potentials, and it stimulates the powerful glial electrogenic Na+-HCO3- cotransporter and other carriers such as Na+-dependent and Na+-independent Cl-/HCO3- exchange (Fig. 3). CO2, as an inert gas, which easily permeates membranes, rapidly dissipates from any location where it is formed through cells and tissues, and together with a high enzyme activity of carbonic anhydrase provides an effective and high buffer capacity even at relatively low concentrations. Considering H+ transients as signals, CO2, HCO3- and carbonic anhydrase are essential elements in this signalling, determining the shape of the pH shifts in time and space within nervous systems.
REFERENCES
Amos, B.J., and Chesler, M. 1998. Characterization of an intracellular alkaline shift in rat astrocytes triggered by metabotropic glutamate receptors. J. Neurophysiol. 79:695-703.
Bevensee, M.O., Apkon, M., and Boron, W.F. 1997. Intracellular pH reguation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J. Gen. Physiol. 110:467-483.
Brune, T., Fetzer, S., Backus, K.H., and Deitmer, J.W. 1994. Evidence for electrogenic sodium-bicarbonate cotransport in cultured rat cerebellar astrocytes. Pflügers Arch. 429:64-71.
Chesler, M. 1990. The regulation and modulation of pH in the nervous system. Progr. Neurobiol. 34:401-427.
Chesler, M., and Kraig, R.P. 1989. Intracellular pH transients of mammalian astrocytes. Neuroscience 9:2011-2019.
Chesler, M., and Kaila, K. 1992. Modulation of pH by neuronal activity. TINS 15:396-403.
Deitmer, J.W. 1991. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J. Gen. Physiol. 98:637-655.
Deitmer, J.W. 1992. Bicarbonate-dependent changes of intracellular sodium and pH in identified leech glial cells. Pflügers Arch. 420:584-589.
Deitmer, J.W., and Schlue, W.R. 1989. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J. Physiol. 411:179-194.
Deitmer, J.W., and Szatkowski, M. 1990. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J. Physiol. 421:617-631.
Deitmer, J.W., Schneider, H.P., and Munsch, T. 1993. Independent changes of intracellular calcium and pH in identified leech glial cells. GLIA 7:299-306.
Deitmer, J.W., and Munsch, T. 1994. Neuron-glia dialogue in the leech central nervous system: glial cell responses to glutamate and kainate. Verh. Dtsch. Zool. Ges. 87.2:185-194.
Deitmer, J.W., and Schneider, H.P. 1995. Voltage-dependent clamp of intracellular pH of identified leech glial cells. J. Physiol. 485.1:157-166.
Deitmer, J. W., and Rose, C.R. 1996. pH regulation and proton signalling by glial cells. Progr. Neurobiol. 48:73-103.
Deitmer, J.W. and Schneider, H.P. 1998. Acid/base transport across the leech giant glial cell membrane at low external bicarobnate concentration. J. Physiol. 512.2:459-469.
Grichtenko, J.J., and Chesler, M. 1994. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience 62:1071-1078.
Hösli, E., and Hösli, J. 1993. Receptors for neurotransmitters on astrocytes in the mammalian central nervous system. Progr. Neurobiol. 40:477-506.
Munsch, T., and Deitmer, J.W. 1994. Sodium-bicarbonate cotransport current in identified leech glial cells. J. Physiol. 474:43-53.
Newman, E.A. 1991. Sodium-bicarbonate cotransport in retinal Müller (glial) cells of the salamander. J. Neuroscience 11:3972-3983.
Rose, C.R., and Deitmer, J.W. 1994. Evidence that glial cells modulate extracellular pH transients induced by neuronal activity in the leech central nervous system. J. Physiol. 481:1-5.
Rose, C.R., and Deitmer, J.W. 1995 Stimulus-evoked changes of extra- and intracellular pH in the leech central nervous system. I. Bicarbonate dependence. J. Neurophysiol. 73:125-131.
Rose, C.R., and Ransom, B.R. 1996. Intracellular sodium homeostasis in rat hippocampal astrocytes. J. Physiol. 419:291-305.
| Discussion Board | Previous Page | Your Symposium |