Invited Symposium: Novel Cellular and Molecular Mechanisms in Allergic Inflammation
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Integrins are a class of heterodimeric cell surface molecules involved in leukocyte adhesion to various immunoglobulin superfamily members as well as matrix proteins (1) . These molecules are composed of an alpha and beta chain and can be grouped into families based on common beta chains, and different leukocytes express different combinations of integrins. For example, the beta-2 integrin family is composed of four known members, CD11a, CD11b, CD11c, and alpha-d, each paired noncovalently with the common beta-2, or CD18, chain (1, 2) . The most recently described member of this family is alpha-d/beta-2. This leukointegrin was initially described in dogs and subsequently in humans (2, 3) , where it is present on most leukocytes in the peripheral circulation (4) . In the study by Van der Vieren, et. al., alpha-d/beta-2 was shown to function as a ligand for ICAM-3, which is an immunoglobulin superfamily member felt to play a role in heterotypic leukocyte interactions (2) . Whether this was the only or most important ligand for alpha-d/beta-2 was unclear.
For some time, we and others have been interested in mechanisms of cell recruitment, with a particular emphasis on the function of adhesion molecules in the selective accumulation of eosinophils during allergic inflammation. One such pathway involves the alpha-4 integrins expressed on eosinophils but not neutrophils. Together, alpha-4/beta-1, and alpha-4/beta-7 are the only previously known ligands for vascular cell adhesion molecule-1 (VCAM-1), and the interaction of alpha-4/beta-1 and alpha-4/beta-7 with VCAM-1 is thought to play a major role in the recruitment of eosinophils to extravascular sites (5-8) . Previously, however, we noted that eosinophil adhesion to VCAM-1 could be partially inhibited by the use of a blocking mAb against CD18, even though at the time none of the three known beta-2 integrins functioned as ligands for VCAM-1 ( (9) and unpublished observations). We therefore hypothesized that alpha-d/beta-2 integrin could act as a ligand for VCAM-1.
Materials and Methods
The following murine IgG1 monoclonal antibodies were used in these studies: irrelevant control IgG1 mAb (Coulter Cytometry, Hileah, FL), alpha-d mAb (169A, non-blocking, used for flow cytometry, and 240I, used in adhesion assays for its blocking ability), alpha-4 blocking mAb (HP2/1, Immunotech, Westbrook, ME), and CD18 blocking mAb (7E4, Immunotech). In the flow cytometry experiments, polyclonal human IgG (Sigma Chemical Co., St. Louis, MO) and an R-phycoerythrin (PE)-conjugated F(ab')2 goat-anti-mouse IgG (BioSource International, Camarillo, CA) were also utilized. For the adhesion studies, soluble recombinant human VCAM-1 (R & D Systems, Minneapolis, MN) and bovine serum albumin (BSA, Sigma) were also purchased.
Normodense eosinophils (s.g. > 1.090) were isolated to > 95% purity from peripheral blood of allergic volunteers by density gradient centrifugation, hypotonic erythrocyte lysis, and immunomagnetic negative selection as previously described (10) . In some experiments, eosinophils were cultured for up to 7 days in RPMI 1640 (Biofluids, Inc., Rockville, MD) with 1% L-glutamine, 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 500 ng/ml amphotericin (Life Technologies, Gaithersburg, MD), and 10 ng/ml recombinant human IL-5 (R & D Systems) as described (11) . Viability at 7 days was >80% as assessed by dye exclusion (11) .
Chinese hamster ovary (CHO) cells were transfected with both the human alpha-d and beta-2 integrin chains as previously described (2) . These cells were cultured in DMEM/F12 media with 1 mM pyruvate and 2 mM L-glutamine (Biofluids, Inc.) supplemented with 10% dialyzed FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 600 µg/ml G418 (Life Technologies). The parental, non-transfected, CHO cells were grown in a similar media, except that non-dialyzed FBS (Life Technologies) was used, and 0.1 mM hypoxanthine and 16 nM thymidine (Sigma) were used in place of the G418.
Flow cytomtetry for determining surface expression of various integrins on eosinophils and CHO cells was performed as previously described (11) .
Adhesion assays were carried out as previously described (9) . Flat 96 well microtiter plates were coated overnight at 4°C with 100µl 1%BSA alone or with soluble recombinant VCAM-1 (250 ng/well). The plate was then washed with Dulbecco's PBS with 1 mM calcium and 1 mM magnesium. For eosinophils, cells were 51Cr-labelled for 30 min prior to addition of mAbs. Then either the eosinophils or CHO cells (not radioactively labelled) were incubated for 30 min at 4°C with appropriate dilutions of the various mAbs, then additional warm (37°C) buffer was added before the cells were aliquotted onto the plate. For some studies, an appropriate dilution of an F(ab')2 anti-VCAM-1 mAb (IG11b1, Caltag Laboratories, Burlingame, CA) was added to certain wells before the addition of the cells. Adhesion was allowed to occur for 30 min at 37°C. In the CHO transfectant cell adhesion assay, nonadherent cells were removed using a modification of a technique described by Schnaar et al (12) , adherent cells collected after treatment with 0.1M EDTA and counted by scatter characteristics on a Coulter EPICS Profile II. In the eosinophil adhesion studies, the plate was washed twice, the remaining adherent cells were lysed and the radioactivity of the lysate determined as previously described (9) . In both cases, percent adhesion was determined by comparing the number of adherent cells to an aliquot of total cells added.
Results
CHO transfectants bind to VCAM-1 via alpha-d/beta-2.
Using CHO cells transfected with both the human alpha-d and beta-2 integrin chains, we performed adhesion assays with immobilized human VCAM-1. As can be seen in the Figure 1 inset, by immunofluorescence and flow cytometry the transfected cells expressed both the alpha-d and beta-2 integrin chains. Non-transfected cells did not express these integrins, and neither cell type expressed any other human beta-2 integrins, alpha-4 integrin, or beta-1 integrin (data not shown). As can be seen in the upper panel of Figure 1, the transfected CHO cells adhered to soluble VCAM-1, and this adhesion was inhibited by an F(ab')2 mAb against the first domain of VCAM-1. This adhesion was also inhibited by mAbs against either the common beta-2 integrin chain (CD18) or alpha-d (Figure 1, lower panel).
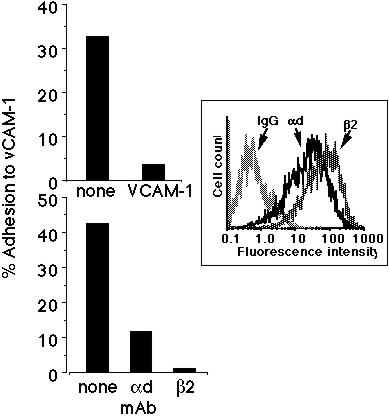 Figure 1: alpha-d/beta-2 transfected CHO cells adhere to VCAM-1. This adhesion is blocked by mAbs against the first domain of VCAM-1 (upper panel), alpha-d integrin, and beta-2 integrin (lower panel). Inset is a representative histogram showing the relative expression of both alpha-d and beta-2 integrin chains on the surface of the transfected CHO cells. Data from a representative experiment are shown (n>3).
Figure 1: alpha-d/beta-2 transfected CHO cells adhere to VCAM-1. This adhesion is blocked by mAbs against the first domain of VCAM-1 (upper panel), alpha-d integrin, and beta-2 integrin (lower panel). Inset is a representative histogram showing the relative expression of both alpha-d and beta-2 integrin chains on the surface of the transfected CHO cells. Data from a representative experiment are shown (n>3).
Expression of alpha-d/beta-2 is augmented on eosinophils cultured with IL-5.
To determine whether eosinophil activation or priming alters expression of alpha-d, cells were cultured with 10 ng/ml recombinant human IL-5 for up to 7 days. Although there was no detectable change in the surface expression of alpha-d in the earlier time points of culture, by 3 days the level of alpha-d began to increase, reaching a statistically elevated level by 4 days (Figure 2 inset and data not shown). In contrast, the surface expression of alpha-4 integrins, the only other known ligands for VCAM-1, remain unchanged throughout the seven days of culture (data not shown).
Eosinophils adhere to VCAM-1 through alpha-d.
We next studied whether freshly isolated human peripheral blood eosinophils and IL-5 cultured eosinophils could adhere to VCAM-1 through alpha-d/beta-2. As can be seen in Figure 2, both freshly isolated eosinophils and 4-7 day IL-5 cultured eosinophils adhered to VCAM-1 coated wells, although the freshly isolated cells had a greater level of adherence than did the cultured ones. Additionally, the background binding was higher in the cultured eosinophils than the freshly isolated cells. Adhesion to VCAM-1 could be inhibited to background levels in the IL-5-cultured cells with a mAb against alpha-d. The same mAb also significantly decreased adhesion in the fresh cells, but to a lesser degree.
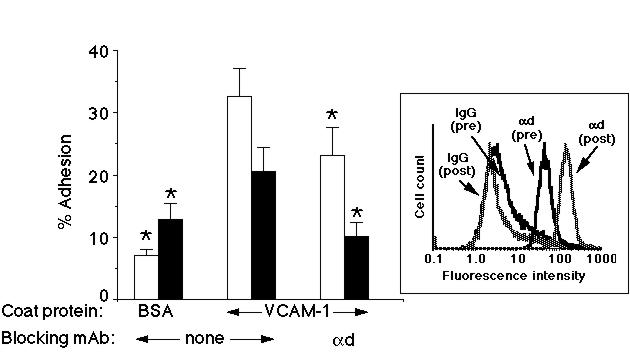 Figure 2: Human eosinophils adhere to VCAM-1 through alpha-d/beta-2 integrin. Adhesion of freshly isolated (open bars) or 4-7 day IL-5 cultured (filled bars) eosinophils can be inhibited by a mAb against the alpha-d integrin. Inset is a representative histogram illustrating the level of alpha-d integrin expression before culture (pre) and after 4 days of culture in IL-5 (post). Data from a representative experiment are shown (n>3).
Figure 2: Human eosinophils adhere to VCAM-1 through alpha-d/beta-2 integrin. Adhesion of freshly isolated (open bars) or 4-7 day IL-5 cultured (filled bars) eosinophils can be inhibited by a mAb against the alpha-d integrin. Inset is a representative histogram illustrating the level of alpha-d integrin expression before culture (pre) and after 4 days of culture in IL-5 (post). Data from a representative experiment are shown (n>3).
Discussion and Conclusion
Using CHO transfectants, we have shown that VCAM-1 is a ligand for alpha-d/beta-2. Interestingly, alpha-4 integrins, the only previously known ligands for VCAM-1, mainly bind to the first domain of VCAM-1 and this interaction is blocked by IG11b1, the anti-VCAM mAb used in this study (13) . This suggests that alpha-d/beta-2, like alpha-4/beta-1 and alpha-4/beta-7, binds to the first domain of VCAM-1. We have also shown that human eosinophils can bind to VCAM-1 through alpha-d/beta-2. Interestingly, the relative importance of this binding seems to increase with IL-5 culture. This parallels an increase in surface expression of alpha-d seen during the culture, while the level of alpha-4 integrins does not change. Whether alpha-d/beta-2 integrin activation, and alpha-4 integrin deactivation (14) , also occurs and contributes to the change in binding seems likely, but is not known.
The importance of alpha-d/beta-2 in eosinophil trafficking is curremtly under investigation. Specifically, the roles of alpha-4 integrins versus alpha-d integrins need to be evaluated. For example, it has been assumed that part of the selective recruitment of eosinophils to sites of allergic inflammation has been due to the fact that they posses alpha-4/beta-1 and alpha-4/beta-7 integrins and neutrophils do not (6, 7) . Neutrophils are not known to bind to VCAM-1; however, they do possess alpha-d/beta-2 integrins (4) . Whether some type of activation is required to allow for alpha-d/beta-2 integrin to bind VCAM-1 in neutrophils is also currently being investigated.
References
- Springer, T. A. 1990. Adhesion receptors of the immune system. Nature 346:425.
- Van der Vieren, M., H. Letrong, C. L. Wood, P. F. Moore, T. St. John, D. E. Staunton, and W. M. Gallatin. 1995. A novel leukointegrin, alpha-d/beta-2, binds preferentially to ICAM-3. Immunity 3:683.
- Danilenko, D. M., P. V. Rossitto, M. Van der Vieren, H. Letrong, S. P. McDonough, V. K. Affolter, and P. F. Moore. 1995. A novel canine leukointegrin, alpha-d/beta-2, is expressed by specific macrophage subpopulations in tissue and a minor CD8(+) lymphocyte subpopulation in peripheral blood. J. Immunol. 155:35.
- Grayson, M. H., M. Van der Vieren, W. M. Gallatin, P. A. Hoffman, and B. S. Bochner. 1997. Expression of a novel beta-2 integrin (alpha-d/beta-2) on human leukocytes and mast cells. J. Allergy Clin. Immunol. 99:S386.
- Bochner, B. S., F. W. Luscinskas, M. A. Gimbrone Jr., W. Newman, S. A. Sterbinsky, C. Derse-Anthony, D. Klunk, and R. P. Schleimer. 1991. Adhesion of human basophils, eosinophils, and neutrophils to IL-1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J. Exp. Med. 173:1553.
- Weller, P. F., T. H. Rand, S. E. Goelz, G. Chi-Rosso, and R. R. Lobb. 1991. Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. USA 88:7430.
- Walsh, G. M., F. A. Symon, A. I. Lazarovits, and A. J. Wardlaw. 1996. Integrin alpha-4/beta-7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology 89:112.
- Bochner, B. S. 1998. Cellular adhesion in inflammation. In Allergy Principles and Practice. 5th Edition. J. E Middleton, C. Reed, E. Ellis, J. NF Adkinson, J. Yunginger, and W. Busse, eds. Mosby, St. Louis, p. 94.
- Matsumoto, K., S. A. Sterbinsky, C. A. Bickel, D. W. Zhou, N. L. Kovach, and B. S. Bochner. 1997. Regulation of alpha-4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1 (VCAM-1). J. Allergy Clin. Immunol. 99:648.
- Hansel, T. T., I. J. M. D. Vries, T. Iff, S. Rihs, M. Wandzilak, S. Betz, K. Blaser, and C. Walker. 1991. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J. Immunol. Methods 145:105.
- Matsumoto, K., R. P. Schleimer, H. Saito, Y. Iikura, and B. S. Bochner. 1995. Induction of apoptosis in human eosinophils by anti-fas antibody treatment in vitro. Blood 86:1437.
- Yang, L. J., C. B. Zeller, and R. L. Schnaar. 1996. Detection and isolation of lectin-transfected COS cells based on cell adhesion to immobilized glycosphingolipids. Anal. Biochem. 236:161.
- Lobb, R. R., and M. E. Hemler. 1994. The pathophysiologic role of alpha-4 integrins in vivo. J. Clin. Invest. 94:1722.
- Werfel, S., T. Yednock, K. Matsumoto, S. A. Sterbinsky, R. P. Schleimer, and B. S. Bochner. 1996. Functional regulation of beta-1 integrins and human eosinophils by divalent cations and cytokines. Am. J. Respir. Cell Mol. Biol. 14:45.
| Discussion Board | Previous Page | Your Symposium |