Cell Biology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Sodium mediates the generation of excitatory synaptic potential and propagation of action potential in mammalian central neurons. Sodium gradient is also used as a source of energy for chemical transport across cell membrane. Persistent elevation in intracellular sodium, such as induced by excitatory glutamate cytotoxicity or epileptic discharge may lead to irreversible cell injury and death. Despite large variations in neuronal excitatory activities associated with multiple routes of sodium entry, most central neurons are capable of maintaining a physiological level of internal sodium.
Central to this sodium buffering capacity is effective expelling of sodium via Na,K-pumping, which is regulated with a Na-dependant feedback mechanism. Upon elevation of internal concentration of sodium, there is increase in sodium binding to catalytic alpha subunit of Na,K-pump which undergoes phosphorylation-induced conformational change. This enhances ion translocation and pump turnover rate.
Recent studies show that besides this kinetic-based modulation, sodium also affects pump mRNA expression and protein synthesis. To address these issues, we have investigated sodium-induced pump regulation in adult rat dissociated thalamic neurons.
Materials and Methods
Experiments were conduced on isolated rat thalamic neurons perfused with warm (32°C) oxygenated Krebs solution. Sodium-channel activator veratridine and sodium-ionophore monensin were used to increase membrane permeability to sodium. Na,K-ATPase inhibitor ouabain and short-acting pump blockers strophanthidin or dihydroouabain were employed to block Na,K-pump activity.
Cell viability was assessed with Calcein AM and Ethidium homodimer. Whole-cell patch-clamp recordings were used to analyse membrane potential and current. Ion imaging was performed with fluorescent probes Sodium GreenTM and Calcium OrangeTM.
Subcellular distribution of alpha-3 catalytic pump subunit was studied using confocal immunofluorescent scanning.
Density of phosphorylated (active) form of Na,K-ATPase on cytoplasmic membrane of alive neurons was assessed using a fluorescent derivative of ouabain, 9-anthroylouabain.
Results
Four-hour viability surveillance of acutely dissociated neurons attested their good health without any significant increase in internal sodium. Neurons have healthy resting and action membrane potentials (Fig.1).
One-hour exposure to veratridine (50 µM) induced slow increase in cell volume: up to 23 ± 7% at the end of four-hour surveillance. Ion imaging of neurons loaded with Sodium GreenTM and Calcium OrangeTM showed that veratridine exposure resulted in progressive rise in internal sodium and calcium with fluorescence increase in 63 ± 24%, and 44 ± 14%, respectively (Fig. 2).
Subcellular confocal immunofluorescent quantification of alpha-3 catalytic pump subunit has detected that veratridine-induced rise in internal calcium was followed by significant increase in the pump density both on membrane and within the cytoplasm: 39 and 54%, respectively (Fig. 3). Incubation with monensin produced similar increase: 28% in membrane domain and 93% in cytoplasmic domain.
Fluorescent 9-anthroylouabain binding assay detected that veratridine exposure results in 60% increase in the membrane density of phosphorylated (active) pump molecules which are composed from a complex of alpha and beta subunits (Fig. 4). Even more significant (110%) fluorescence enhancement was found in neurons incubated with Na-ionophore monensin.
Impairment of Na,K-ATPase activity with ouabain during veratridine exposure resulted in even higher increase in internal sodium and calcium (fluorescence increase up to 85 ± 29% and 193 ± 61%, respectively), cell swelling with volume increase up to 66 ± 20%, and massive neuronal death
The major finding reported in this paper is the feedback signalling mechanism for a regulation of the Na,K-ATPase expression by which the central mammalian neurons can fight sodium overload. Our data demonstrate that persistent activation of sodium channels results in significant up-regulation of the Na,K-ATPase in adult rat central neurons. Ion imaging data show that up-regulation of Na,K-ATPas is preceded by and associated with a progressive rise in intracellular sodium. The fact that neurons survive veratridine exposure but die quickly from simultaneous exposure to veratridine and ouabain suggests a physiological role for Na,K-ATPase up-regulation as a neuroprotective mechanism against sodium cytotoxicity.
It is concluded that mammalian central neurons adopt limited capacity for Na-mediated feedback regulation of pump activity and synthesis of new molecules, which may moderate rise in internal sodium and delay cell death following persistent activation of sodium channels.
 Click to enlarge
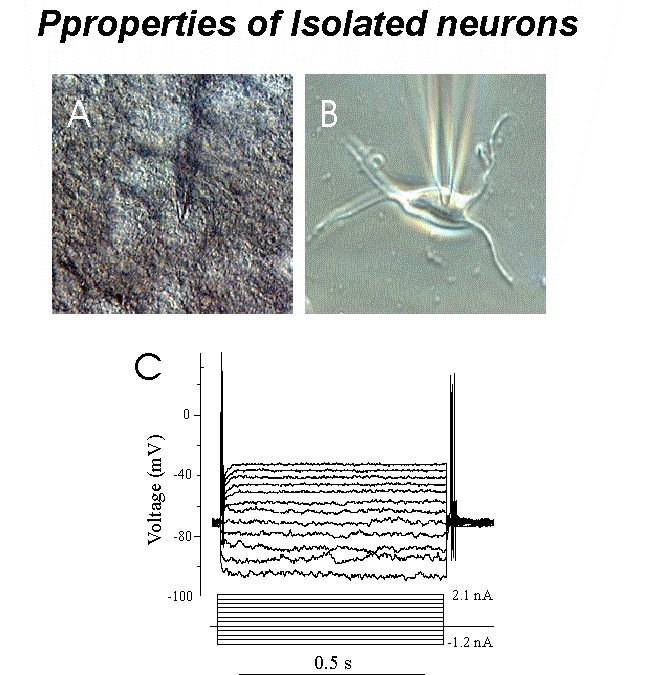
Fig. 1: Isolated thalamic neurons show healthy structural and physiological properties. A: Hoffman modulation contrast image of neuron during patch-clamp recording from thalamic slice. B: Phase-contrast image of representative isolated neuron during patch-clamp recording. Note that isolated neuron preserves the same shape as in situ. C: Membrane voltage traces induced by de- and hyperpolarizing current pulses injected into the neuron shown in (B). Note robust membrane resting and action potentials.
Click to enlarge
Fig. 1: Isolated thalamic neurons show healthy structural and physiological properties. A: Hoffman modulation contrast image of neuron during patch-clamp recording from thalamic slice. B: Phase-contrast image of representative isolated neuron during patch-clamp recording. Note that isolated neuron preserves the same shape as in situ. C: Membrane voltage traces induced by de- and hyperpolarizing current pulses injected into the neuron shown in (B). Note robust membrane resting and action potentials.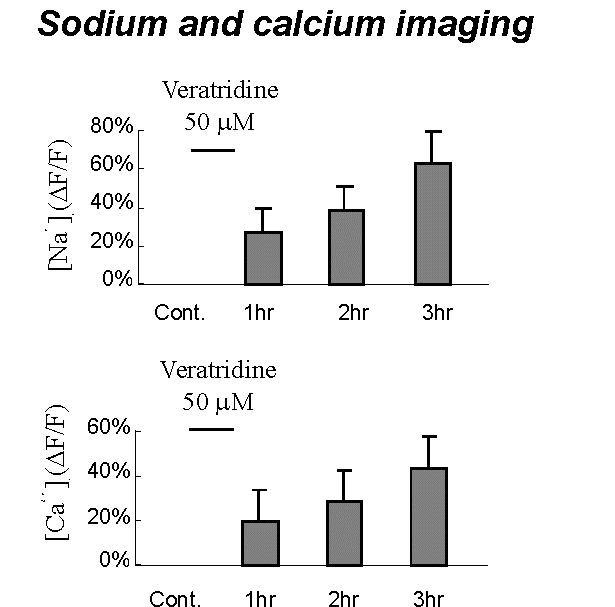 Click to enlarge
Fig. 2: Results of sodium and calcium imaging obtain from group of neurons after one-hour exposure to 50 µM veratridine.
Click to enlarge
Fig. 2: Results of sodium and calcium imaging obtain from group of neurons after one-hour exposure to 50 µM veratridine.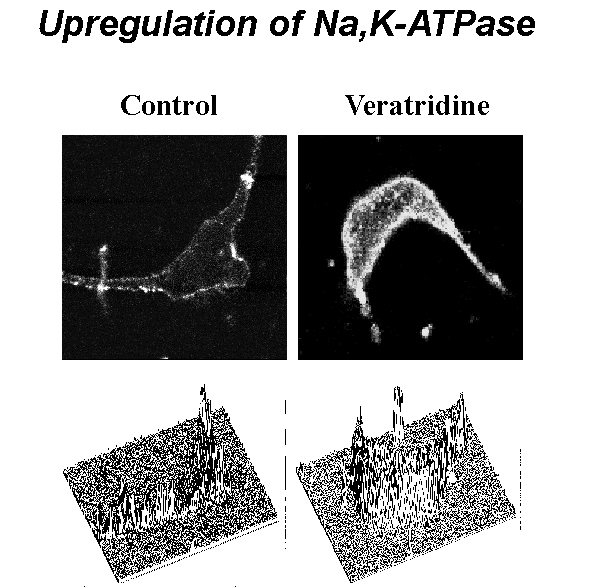 Click to enlarge
Fig. 3: Confocal scanning of neurons stained with fluorescent antibody revealed significant increase in the density of alpha-3 subunit. Fluorescence images (top) and 3D intensity profiles (bottom) of two representitative neurons - control (left) and exposured to veratridine (right).
Click to enlarge
Fig. 3: Confocal scanning of neurons stained with fluorescent antibody revealed significant increase in the density of alpha-3 subunit. Fluorescence images (top) and 3D intensity profiles (bottom) of two representitative neurons - control (left) and exposured to veratridine (right). 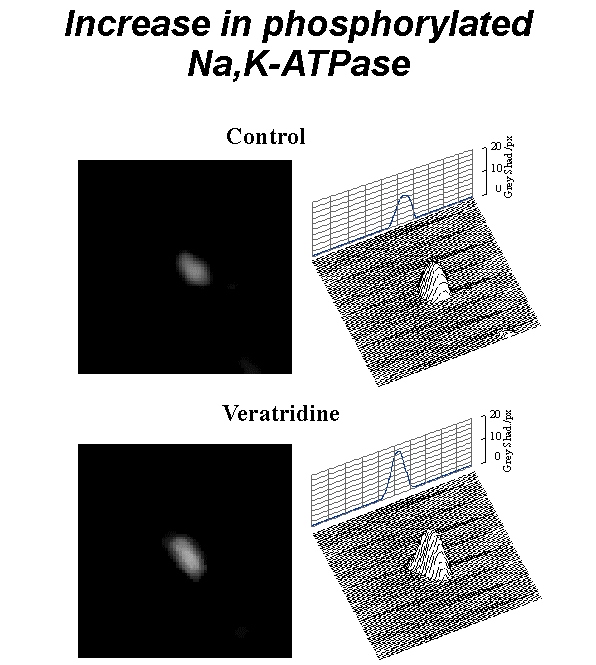 Click to enlarge
Fig. 4: Increase in the membrane density of phosphorylated (active) pump molecules after exposure to veratridine (50 µM). Fluorescent images, and 3D intensity profiles of two representitative neurons - control (top) and exposed to veratridine (bottom).
Click to enlarge
Fig. 4: Increase in the membrane density of phosphorylated (active) pump molecules after exposure to veratridine (50 µM). Fluorescent images, and 3D intensity profiles of two representitative neurons - control (top) and exposed to veratridine (bottom).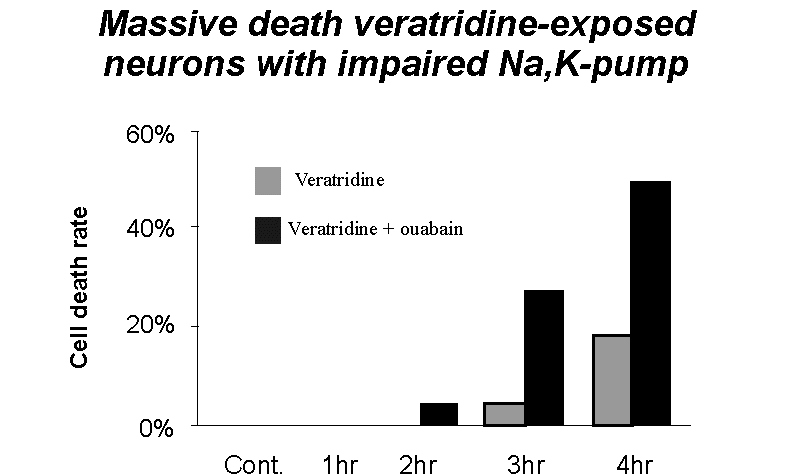 Click to enlarge
Fig. 5: Comparative results of cell viability assay after exposure to veratridine alone (50 µM) or in combination with ouabain (250 µM). Note significant increase in cell death after simultaneous inhibition of the Na,K-pump.
Click to enlarge
Fig. 5: Comparative results of cell viability assay after exposure to veratridine alone (50 µM) or in combination with ouabain (250 µM). Note significant increase in cell death after simultaneous inhibition of the Na,K-pump.
Discussion and Conclusion
| Discussion Board | Previous Page | Your Poster Session |