Cell Biology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Cardiac hypertrophy following thyroxine administration to experimental animals is associated with increased velocity of contraction and speed of diastolic relaxation which is thought to result from changes in the velocity of cytoplasmic Ca2+ sequestration by the sarcoplasmic reticulum (SR) (1). Ca2+-ATPase of SR performs the essential function of promoting muscle relaxation by rapidly removing Ca2+ from the cytosol (2) and its function is well known to be regulated by the phosphorylation of another cardiac SR-specific protein called phospholamban (3). Evidence from recent studies have suggested a crucial role for Ca2+/calmodulin-dependent protein kinase II (CaM kinase) in the regulation of SR function in heart muscle (4) apparently through phosphorylation of SR proteins Ca2+-ATPase and phospholamban which are involved in transmembrane Ca2+ cycling.
To date, observations supporting thyroid hormone-induced alternations in SR function include increased Ca2+ uptake by SR isolated from hearts with thyrotoxic hypertrophy (5,6), elevated cardiac mRNA level of SR Ca2+-ATPase and decreased mRNA level of phospholamban following thyroid hormone treatment (7,8). The present study is aimed to determine the impact of L-thyroxine-induced hyperthyroidism on CaM kinase mediated SR protein phosphorylation and SR Ca2+ pump activity in cardiac and slow-twitch skeletal muscle (soleus) of the rabbit. The results obtained demonstrate that (a) overexpression of different SR Ca2+-ATPase isoforms contributes to the enhanced SR Ca2+ sequestration in hyperthyroid heart and slow-twitch soleus muscle; (b) the expression of Ca2+-ATPase and PLN are not coordinately regulated in both tissues.
Materials and Methods
METHODS
Induction of hyperthyroidism in rabbits and preparation of SR membranes:
The studies were performed using SR membrane vesicles isolated from cardiac muscle and slow-twitch skeletal muscle (soleus) of euthyroid and hyperthyroid NewZealand White male rabbits. Hyperthyroidism, with attendant cardiac hypertrophy, was induced by the injection of L-thyroxin (200 mg/kg body weight) daily for 7 days. SR membrane vesicles from cardiac and soleus muscle were isolated as described previously (11). Cardiac SR vesicles were subfractionated into membrane vesicles enriched in junctional SR (JSR) and longitudinal SR (LSR) by density gradient centrifugation according to the procedure of Gasser etal.
Western immunoblotting and phosphorylation assay:
Western immunoblotting analysis of SERCA1 and SERCA2 Ca2+-ATPase isoforms and phospholamban using monoclonal antibodies specific for these proteins according to the procedures detailed elsewhere (4). Phosphorylation of SR proteins by endogenous CaM kinase was determined as described previously (3). Phosphorylation assay medium (total volume 50 ml) contained 50 mM HEPES (pH 7.4), 10 mM MgCl2, 2 mM CaCl2, 2 mM calmodulin, 0.8 mM [g-32P]ATP (specific activity 300-400 cpm/mol), and SR (20-25 mg of protein). The phosphorylation reaction was initiated by addition of [g-32p]ATP following preincubation of the rest of assay components for 3 min at 37°C. The Ca2+/calmodulin-dependence of phosphorylation was monitored in parallel assays lacking Ca2+ (1 mM EGTA present) and calmodulin in the assay medium. Reactions were terminated after 2 min by addition of 15 ml of SDS sample buffer and the samples were subjected to SDS-PAGE in 4-18% gradient gel, stained with commassie Blue, dried and autoradiographed. Quantification of phosphorylation was carried out by liquid scintillation counting of radioactive bands excised from the gels.
Ca2+ uptake assay:
ATP-dependent oxalate-facilitated Ca2+ uptake by SR was determined using the Millipore filtration technique (11). The standard incubation system (total volume 250 ml) contained 50 mM Tris-maleate (PH=6.8), 5 mM NaN3, 5 mM ATP, 5mM MgCl2, 120 mM KCl, 0.1 mM EGTA, 25 mM ruthenium red, 5mM potassium oxalate, SR vesicles (20-30 mg of protein) and varying concentrations of 45CaCl2 (~8,000 cpm/nmol). The Ca2+ uptake reaction was initiated by addition of ATP following preincubation of the rest of the assay components for 3 min at 37°C. The initial free Ca2+ concentrations in the assay mediums was determined using computer program of Fabiato (13). Data Presentation: The results are presented as means ± SE. Statistical significance was evaluated by the Student's t test; p< 0.05 was taken as the level of significance.
Results
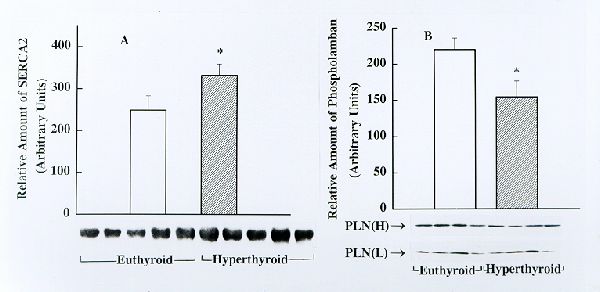
Fig. 1. Detection and estimation of the relative amounts of SERCA2 (panel A) and phospholamban (PLN, panel B) in cardiac junctional SR (JSR) from euthyroid and hyperthyroid rabbits by Western blotting. Identical amounts of cardiac JSR (30 mg protein) from euthyroid and hyperthyroid rabbits were subjected to Western immunoblotting analysis using SERCA2- and phospholamban-specific monoclonal antibodies. Immunoblots obtained using several JSR preparations from euthyroid and hyperthyroid rabbits are shown on the bottom. The bar graphs depict the relative amount of immunnoreactive protein (presented as mean± SE) as determinated by densitometric scanning of Western blots. Note that the amount of SERCA2 is significantly higher and phospholamban is significantly lower, in JSR from hyperthyroid compared to that from euthyroid ( * p<0.05 )
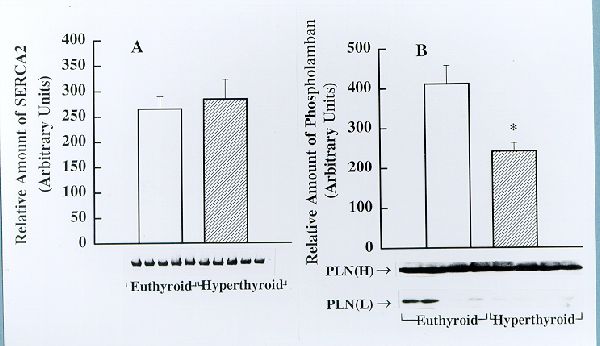
Fig. 2. Detection and estimation of the relative amounts of SERCA2 (panel A) and phospholamban (PLN, panel B) in cardiac longitudianal SR (LSR) from euthyroid and hyperthyroid rabbits by Western blotting. Identical amounts of cardiac LSR (30 mg protein) from euthyroid and hyperthyroid rabbits were subjected to Western immunoblotting analysis using SERCA2- and phospholamban- specific monoclonal antibodies. Immunoblots obtained using several LSR preparations from euthyroid and hyperthyroid rabbits are shown on the bottom. The bar graphs depict the relative amount of immunnoreactive protein (presented as mean± SE) as determinated by densitometric scanning of Western blots. Note that the amount of phospholamban is significantly decreased in JSR from hyperthyroid compared to that from euthyroid (* p<0.05 ); however, there is no significant difference in the amount of SERCA2 between the two groups.
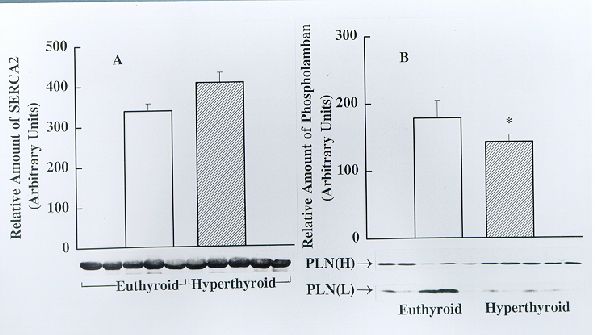
Fig. 3. Detection and estimation of the relative amounts of SERCA2 (panel A) and phospholamban (PLN, panel B) in soleus muscle SR from euthyroid and hyperthyroid rabbits by Western blotting. Identical amounts of SR protein (30 mg) from euthyroid and hyperthyroid rabbits were subjected to Western immunoblotting analysis using SERCA2- and PLN- specific monoclonal antibodies. Immunoblots obtained using five separate SR preparations each from euthyroid and hyperthyroid rabbits are shown on the bottom. The bar graphs depict the relative amount of immunoreactive protein (presented as mean ± SE) as determined by densitometric scanning of Western blots. Note that the amount of SERCA2 does not differ significantly between euthyroid and hyperthyroid whereas the amount of PLN is significantly lower (*p<0.05) in the hyperthyroid.
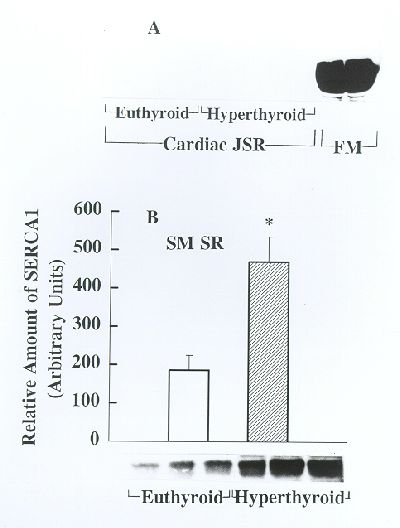
Fig. 4. Analysis of SERCA1 expression in cardiac and soleus muscle SR from euthyroid and hyperthyroid rabbits by Western blotting. Identical amounts of cardiac JSR and soleus muscle SR from euthyroid and hyperthyroid rabbits were subjected to Western immunoblotting analysis using SERCA1-specific monoclonal antibodies. SR from fast-twitch skeletal muscle (FM) from an euthyroid rabbit was used as a positive control. Panel A shows Western blots obtained using SR from cardiac muscle and FM. Representative Western blots obtained for soleus muscle SR are shown on bottom of panel B and the bar graph depicts the relative amount of immunoreactive protein (presented as mean ± SE, n=5) as determined by densitometric scanning of Western blots. Note that SERCA1 is not detectable in cardiac SR from euthyroid and hyperthyroid groups. In contrast, there is a striking overexpression of SERCA1 in soleus muscle SR from the hyperthyroid compared to euthyroid (*p<0.05).
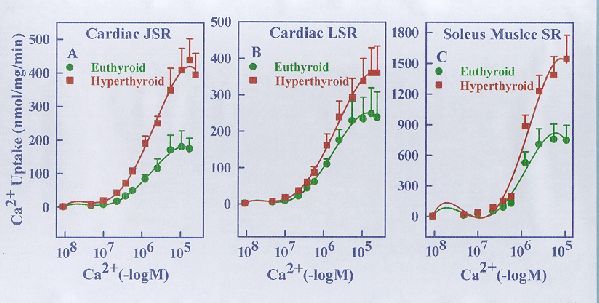
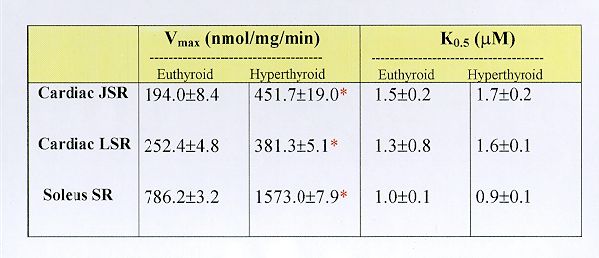
Fig. 5. Ca2+ concentration dependence of ATP-supported Ca2+ uptake by cardiac JSR (panel A), cardiac LSR (panl B) and soleus muscle SR (panel C) from euthyroid and hyperthyroid rabbits. The Ca2+ uptake reaction was carried out under the standard assay conditions (see Methods) in the presence of varying concentrations of free Ca2+ as indicated. Each data point represents the mean ± SE of five experiments using separate membrane preparations. The kinetic parameters derived from these data are shown in Table 1 below. Note that the Vmax of Ca2+ transport but not the K0.5 for Ca2+ is altered significantly in the case of SR membranes from hyperthyroid compared to euthyroid (*p<0.05).
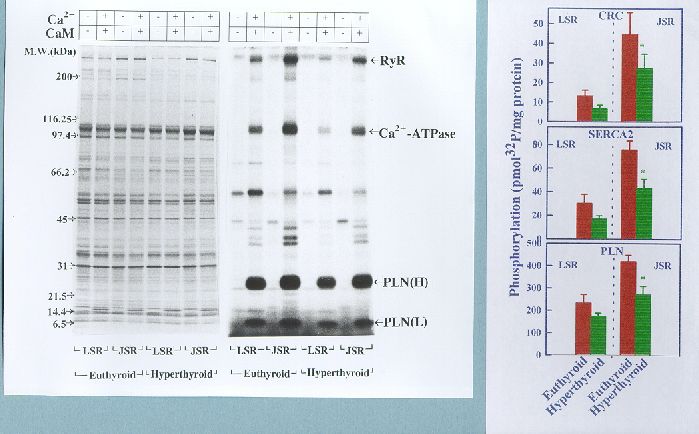
Fig. 6. Comparison of endogenous CaM kinase-mediated phosphorylation of Ca2+-cycling proteins in LSR and JSR of euthyroid and hyperthyroid rabbit hearts. The phosphorylation reaction was carried out for 2 min in the absence (-) and presence (+) of Ca2+/ calmodulin (CaM) as indicated. Left panel shows Commassie Blue-stained SDS-polyacrylamide gel showing SR protein profiles. Right panel shows autoradiogram of the same gel. The data shown in bar graphs compare phosphorylation of the substrate proteins as quantified by liquid scintillation counting of the peptide bands excised from SDS-PAGE gels (n=5). RYR, ryanodine receptor; PLN(H), high molecular weight form of phospholamban; PLN(L), low molecular weight form of phospholamban; Note that the Ca2+/calmodulin-dependent phosphorylation of RYR, Ca2+-ATPase and phospholamban is decreased significantly in the hyperthyroid (*p<0.05) compared to euthyroid.
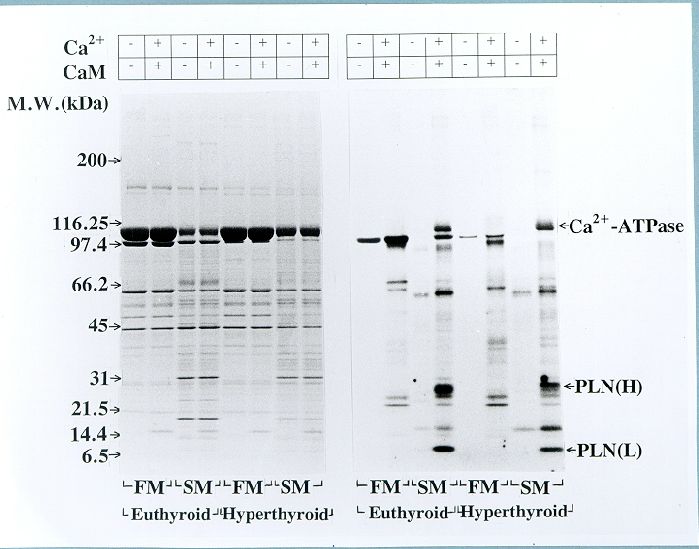
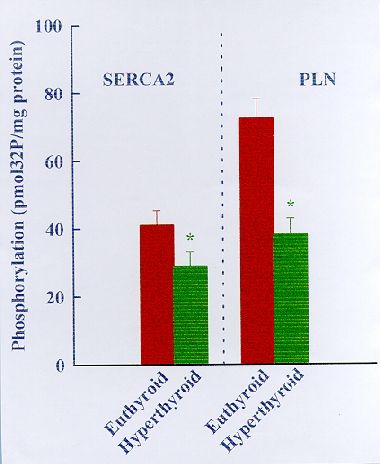
Fig. 7. Comparison of endogenous CaM kinase mediated protein phosphorylation in fast-twitch muscle (FM) SR and soleus muscle (SM) SR from euthyroid and hyperthyroid rabbits. The phosphorylation reaction was carried out for 2 min in the absence (-) and presence (+) of Ca2+ and calmodulin (CaM) as indicated. Left panel shows Coomassie Blue-stained SDS-polyacrylamide gel showing SR protein profiles. Right panel shows autoradiograqm of the same gel. The data shown in the bar graph compare phosphorylation of Ca2+-ATPase (SERCA2) and phospholamban (PLN) in SM SR as quantified by liquid scintillation counting of the peptide bands excised from SDS-PAGE gels (n=5). Note that the Ca2+/CaM-dependent phosphorylation of Ca2+-ATPase and PLN is decreased significantly in SM SR from hyperthyroid compared to euthyroid (*P<0.05).
Discussion and Conclusion
CONCLUSIONS
1. In the rabbit, L-thyroxine-induced hyperthyroidism is associated with overexpression of SERCA2 and SERCA1 Ca2+-ATPase isoforms in the heart and slow-twitch soleus muscle, respectively.
2. Expression of the Ca2+-ATPase regulatory protein phospholamban is significantly reduced in both cardiac and soleus muscles of the hyperthyroid compared to euthyroid rabbit.
3. SR vedicles from cardiac and soleus muscles of hyperthyroid rabbits display significantly higher rates of ATP-energized Ca2+ sequestration (Ca2+ pump activity) when compared to their euthyroid counterparts.
4. Endogenous CaM kinase mediated phosphorylation of phospholamban and Ca2+-ATPase is significantly lower in cardiac and soleus SR from the hyperthyroid compared to euthyroid rabbit.
5. It is concluded that (a) overexpression of different SR Ca2+-ATPase isoforms contributes to the enhanced SR Ca2+ sequestration in hyperthyroid heart and slow-twitch soleus muscle; (b) the expression of Ca2+-ATPase and phospholamban are not coordinately regulated in both
References
1.Alpert N.R., Blanchard E.M., and Mulieri L.A. (1987) In: Pathophysiology of Heart Disease (Dhalla, N.S., Singal, P. and Beamish, R., eds.) pp 99-111
2. Davies B.A., Edes I., Gupta R.C., Young E.F., Kim H.W., Steenart N.A.E. Szymanska G., and Kranias E.G. (1990) Mol.Cell.Biochem. 99:83-88
3. Xu A., Hawkins C., Narayanan N. (1993) J. Biol. Chem. 268: 8394-8397
4. Xu A., Hawkins C., Narayanan N. (1997) J. Mol.Cell.Cardiol. 29:405-418
5. Hain J., Onoue H., Mayrleitner M., Fleischer S. Schindler H (1995) J. Biol. Chem. 270:2074-2081
6. Witcher D.R., Kovacs R.J., Schulman H., Cefali D.C. Jones L.R. (1991) J. Biol. Chem. 266: 11144-11152
7. Suko J. (1973) J. Physiol. London 228: 563-582
8. Limas C. J. (1978) Am. J. Physiol 235: H745-H751
9. Arai M., Otsu K., MacLennan D.H., Alpert N.R., and Periasamy M. (1991) Circ. Res. 69: 266-276
10. Rohrer D. and Dillman W.H. (1988) J. Biol. Chem. 263:6941-6944
11. Narayanan N. (1981) Biochim. Biophys. Acta. 678:442-459
12. Gasser J. Paganetti P., Carafoli E., Chiesi M. (1988) Eur. J. Biochem. 176: 535-541
13. Fabito, A. (1988) Methods Enzymol. 157: 378-417
| Discussion Board | Previous Page | Your Poster Session |