Invited Symposium: Neural Mechanism of Mammalian Vocalization
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Speech is one of the most remarkable features of the human species, and is the object of study of many scientists of different disciplines, such as the humanities, arts, medical and biological sciences. Almost all these studies concern the most "human" part of speech, i.e. the skill to produce words and sentences as well as the skill to memorize how to produce these words, i.e. to reproduce words and sentences at a very high speed and with great precision. Not only forming a word is a typical example of cerebral cortical activity, but also the ability, after hearing spoken words, to understand and memorize the meaning of them. The basic concept brought forward is this paper is that for the production of speech, two motor systems play a role, the somatic and the emotional motor system (Figure 1).
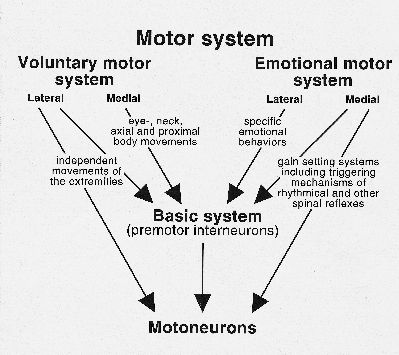 Fig. 1: Schematic overview of the basic, voluntary and emotional divisions of the motor system.
Fig. 1: Schematic overview of the basic, voluntary and emotional divisions of the motor system.
The somatic motor system
The somatic motor system can be subdivided into a medial and a lateral system. The medial system in mammals is involved in the maintenance of erect posture (antigravity movements), integration of body and limbs, synergy of the whole limb and orientation of body and head,(for review, see Kuypers, 1981).
The lateral system, on the other hand, serves as the motor control system for distal limb movements. In monkeys, apes and humans the corticospinal system is the most important component of the lateral system, and its direct projections to motoneurons enables monkeys and primates to perform complicated independent finger and other distal movements. The lateral system is also of vital importance for the precise control of speech muscles. In humans the face part of the motor cortex projects to the premotor interneurons in the lateral tegmental field of the caudal brainstem, but also directly to the motoneuronal cell groups innervating mouth, tongue and pharynx/larynx (Kuypers, 1958). Interruption of these fibers results in volitional facial paresis.
It is common knowledge that the left cortex cerebri is crucial for speech. In the many patients with left sided cerebrovascular accidents normal speech is no longer possible. A major component of the cortical speech production are the direct cortical projections to speech motoneurons in the caudal brainstem. Before the primary motor cortex neurons send signals to the brainstem motoneurons, they receive detailed commands from premotor regions of the cortex. Well known in this respect is the so-called area of Broca (area 45), which sends many fibers to the corticobulbar and corticospinal neurons in the primary motor cortex (area 4). Such an extensive premotor area for producing speech can only be found in humans. It might be regarded as the motor memory of speech. In simple terms one might hypothesize that the strategy, necessary to perform a certain motor activity, such as the pronunciation of a word, once successfully executed, is memorized in the premotor cortex, and in case of speech, in the premotor speech area or area of Broca. When the same word has to be pronunciated another time, not all the cortical association areas are necessary as when it was pronunciated the first time. For making words it is not sufficient to produce a certain consonant or vowel, but the individual has to remember also which consonant or vowel comes first, which next and so on. This all has to occur at an extremely high speed, so that extensive motor memory is needed, which explains why Broca’s area occupies such a large portion of the cortex. It has to memorize the motor strategy to produce the right sequence of consonants and vowels for a large number of words. Learning all these difficult tasks takes a long time, and children need several years to reach the ability to pronounce all the words properly.
The emotional motor system (EMS)
The EMS, similar to the somatic motor system, uses the motoneurons and the premotor interneurons (basic motor system according to the scheme of Fig. 1) as tools for their motor output. Similar to the somatic motor system, the EMS consists of a medial and a lateral component. Both components use the basic system premotor interneurons to reach the motoneurons. Only the medial component has direct access to motoneurons. It consists of an extremely diffuse descending system, which is involved in so-called level setting of motoneurons. For further review of the brain regions involved, see Holstege, (1991; 1997). Although the motoneurons innervating larynx, pharynx, abdominal, and other speech related muscles also receive afferents from systems belonging to the medial component of the EMS, it is unlikely that these systems play a specific role in speech production.
The lateral component of the EMS does not project directly to motoneurons, but uses basic system premotor interneuronal cell groups as relay. Fig. 2 gives an overview of the mesencephalic descending pathways, with their possible functions. The pathways involved in micturition, blood pressure, lordosis, vocalization and defensive posture belong to the lateral component of the EMS, and they all make use of premotor interneuronal cell groups.
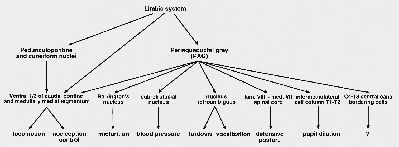 Fig. 2: Schematic overview of the descending pathways from the mesencephalon to caudal brainstem and spinal cord, and the possible functions these pathways might be involved.
Fig. 2: Schematic overview of the descending pathways from the mesencephalon to caudal brainstem and spinal cord, and the possible functions these pathways might be involved.
In respect to animal vocalization it has been shown that it can be elicited in various limbic structures as the lateral bed nucleus of the stria terminalis, central nucleus of the amygdala, lateral hypothalamus, as well as in the frontal cortex (Jürgens and Ploog, 1970; Price, 1996) it is most easily elicited in the caudal PAG (see Holstege, 1989 for review). The fact that bilateral lesions in this region produce muteness (Adametz and O'Leary, 1959, Jürgens and Pratt, 1979) is a further indication of the strong involvement of the PAG in vocalization. Apparently, the final integration of the motor act of vocalization takes place in the PAG. Holstege (1989) in the cat suggested that there exists a specific pathway for the PAG to produce vocalization. This pathway originates from neurons within the lateral and dorsal parts of the caudal PAG. Cells in these regions specifically project to the nucleus retroambiguus (NRA) and adjoining lateral tegmental field. Later studies have revealed that indeed the NRA plays a crucial role in vocalization, because transections at -1 mm caudal to the obex did abolish vocalization elicited in the periaqueductal gray (PAG), whereas vocalization was not affected after transections at -4 mm caudal to the obex (Zhang et al., 1995).
Role of the nucleus retroambiguus (NRA)
The NRA is a premotor interneuronal cell group in the caudal medulla oblongata. A lightmicroscopical axonal tracing study of Holstege (1989) has revealed that the NRA cells project mainly contralaterally to the motoneuronal cell group of pharynx and soft palate. However, the NRA also projects to the motoneurons of internal intercostal and abdominal muscles (in the hamster Gerrits and Holstege, 1998; in the rat Holstege et al. 1997; in the cat Holstege and Kuypers, 1982 and Holstege, 1989; in the monkey VanderHorst et al. 1998).
Pharynx and soft palate motoneurons are located in the only portion of the nucleus ambiguus that, in Nissl-stained sections of the medulla oblongata, can be distinguished as a separate nucleus. Holstege’s (1989) study did not allow to demonstrate NRA projections to larynx motoneurons, because they do not form a distinct nucleus, but are scattered in the ventrolateral reticular formation of the medulla oblongata between the level of the obex and the facial nucleus (Davis and Nail, 1984). In order to solve this problem of larynx motoneuronal afferents, a recent study in the monkey (VanderHorst, in preparation) have combined retrogradely labeled larynx motoneurons with anterogradely labeled NRA axons. The electronmicroscopic results demonstrate that the NRA has a direct excitatory projection to larynx motoneurons. In respect to the NRA projections to abdominal and internal intercostal muscles, recent double labeling electronmicroscopic studies in cat and monkey (Boers, VanderHorst and Holstege, in preparation) demonstrated direct excitatory NRA fibers terminating on external oblique abdominal muscle motoneurons. Contraction of the abdominal and internal intercostal muscles causes an increase in the abdominal pressure necessary for generating vocalization.
Although the larynx, pharynx, and soft palate are involved in increasing abdominal pressure, they also play a role in generating different vocalizations. Animals use various kinds of vocalizations, depending on the circumstances (threat, immobility, sexual activities and so on.). These different vocalizations are embedded in the total organization of survival behavior organized in the PAG. For example, as shown by Bandler et al. (1991) stimulation in the lateral part of the pretentorial PAG by means of excitatory amino acids (EAA) elicits hissing like vocalizations in the framework of moderate threat display. On the other hand, similar EAA stimulation in the subtentorial PAG results in howling like vocalizations in the framework of strong threat display. Stimulation in the most caudal PAG did not elicit hissing or howling, but bursts of hindlimb movements, belonging to strong flight reactions. In contrast to the lateral portions of the lateral PAG, EAA stimulation in the most ventral portions of the lateral PAG did not elicit vocalizations, but immobility of the animal, accompanied by low arterial pressure (Bandler et al. 1991). In other words, vocalization is not a separate function of the central nervous system of which the final organization takes place in the PAG, but it is one of the many components of the basic survival behaviors that are generated in the PAG (Fig. 1).
An interesting example of a function other than vocalization generated in the PAG is copulation behavior. It has been known for some time that the PAG is the final integrator for so-called lordosis behavior in rats (Sakuma and Pfaff, 1979). Recent findings of VanderHorst and Holstege (1995) show that the PAG, in order to execute the copulation movements, uses the same premotor interneuronal cell group (NRA) as for vocalization (Fig. 3). These findings suggest the NRA contains different kinds of interneurons.
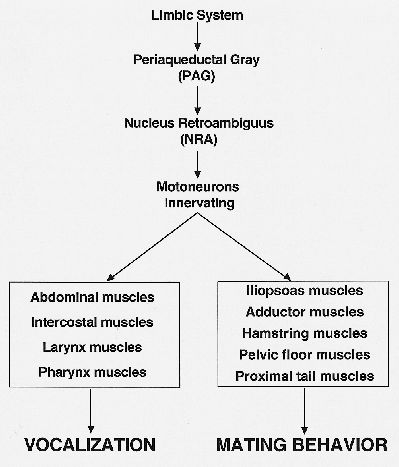 Fig. 3: Schematic representation of the concept for the final common pathways for vocalization and lordosis.
Fig. 3: Schematic representation of the concept for the final common pathways for vocalization and lordosis.
Two concepts about the organization of the NRA According to the first concept one NRA cell projects to only one specific group of motoneurons, i.e. to the motoneurons of respectively the pharynx, larynx, abdominal, intercostal, hindlimb or certain other muscle motoneurons. In that case the PAG determines the motor activity to be executed by activating specific combinations of NRA cells, belonging to certain combinations of muscle motoneurons. Via its pathways to the NRA, the PAG can generate vomiting, vocalization, copulation or other movements, or even combinations of these activities.
According to the second concept, the NRA cells are exclusively involved in certain functions, i.e. one portion of the NRA cells project to the motoneurons involved in vocalization, another portion of NRA cells project to the motoneurons involved in copulation behavior and a further group of NRA cells activate the motoneurons involved in the act of vomiting. In this concept one NRA cell will have contacts with several motoneuronal cell groups. For example, a vocalization involved NRA neuron will have connections with pharynx, larynx, intercostal, as well as with abdominal muscle motoneurons, but not with hindlimb motoneurons. NRA cells that are concerned with copulation movements have projections to the hindlimb, certain axial and possibly abdominal muscle motoneurons, but not to motoneurons of pharynx and larynx. Within this second concept, one might even expect that some NRA cells specifically involved in hissing like vocalizations and others in mewing or howling. Such cells differ slightly in their projections to the various pharynx and larynx motoneurons. It is not known which of the two concept is true, and might also be possible that the NRA organization is a combination of the two.
In respect to future research, the findings of VanderHorst and Holstege, (1997) are of interest. They observed strong effects of estrogen on the NRA terminations on hindlimb motoneurons. These terminations become almost ten times as numerous in estrous than in non-estrous cats. It might be possible that similar changes occur in the NRA projections to the pharynx and larynx motoneurons, which could explain why vocalization in estrous cats is so different from that in non-estrous cats.
Speech as the product of somatic and emotional motor systems
From anatomical and physiological studies it is clear that the somatic motor system, i.e. the motor cortex, maintains direct projections to the speech motoneuronal cell groups. Lesions in this pathway results in inability to speak (motor aphasia), but aphasic patients can still vocalize. Possibly, based on anatomical and physiological findings obtained in the cat, vocalization in these patients might be possible because of an intact PAG-NRA-motoneuronal pathway. Clinical cases with lesions in the caudal PAG, not extending into surrounding areas leading to conditions as akinetic mutism, are scarce. However, a few human cases have been described (Botez and Barbeau, 1957), suggesting that after lesions in the PAG, the production of sound, including speech, is no longer possible.
Perhaps, during speech the emotional motor system is basic for the production of sound, but the direct cortico-motoneuronal pathways, controlled by Broca’s area, modulate this vocalization. In other words, the PAG-NRA-motoneuronal pathway is responsible for the sound production, and the cortico-motoneuronal pathway for the production of specific vowels or syllables (Fig. 4). The result is speech. In respect to singing, a similar organization exists, with the emotional motor system producing the basic sound, but with certain cortical areas modulating sound into song.
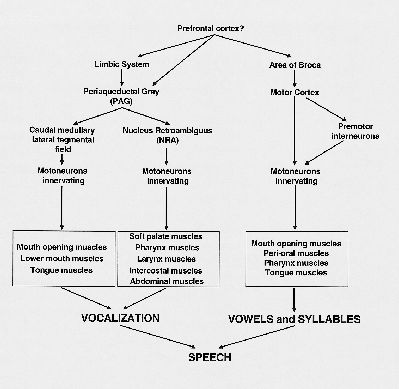 Fig. 4: Schematic overview of the pathways possibly involved in the production of speech.
Fig. 4: Schematic overview of the pathways possibly involved in the production of speech.
References
Adametz J., and J. L. O'Leary (1959) Experimental mutism resulting from periaqueductal lesions in cats. Neurology 9, 636-642
Bandler, R., Carrive, P. and Zhang, S.P. (1991) Integration of somatic and autonomic reactions within the midbrain periaqueaductal grey: Viscerotopic, somatotopic and functional organization. In: "Role of the forebrain in sensation and behavior" G. Holstege (Ed.) Elsevier Amsterdam, Progr. Brain Res. Vol. 87: p.269-305
Botez M.I., and A. Barbeau (1971) Role of subcortical structures and particularly of the thalamus, in the mechanisms of speech and language. Int. J. Neurol. 8, 300-320
Davis J., and B. S. Nail (1984) On the location and size of laryngeal motoneurons in the cat and rabbit J. Comp. Neurol. 230, 13-32
Gerrits, P.O., and Holstege, G. (1998) Descending projections from the nucleus retroambiguus to the iliopsoas motoneuronal cell groups in the female golden hamster; possible role in reproductive behavior. J. Comp. Neurol. in press
Holstege G. (1989) An anatomical study on the final common pathway for vocalization in the cat J. Comp. Neurol. 284, 242-252
Holstege G. (1991) Descending motor pathways and the spinal motor system: limbic and non-limbic components. In: "Role of the forebrain in sensation and behavior" G. Holstege (Ed.) Elsevier Amsterdam, Progr. Brain Res. Vol. 87, p. 307-412
Holstege, G. (1997) The emotional motor system. In the Encyclopedia of Human Biology second edition Academic Press Inc. San Diego volume 3: 643-660
Holstege, G., Moes, M.C., Kerstens, L. and VanderHorst, V.G.J.M. (1997) Evidence for a periaqueductal gray - nucleus retroambiguus - spinal cord pathway in the rat. Neuroscience, 80: 587-598
Jürgens, U., and Ploog, D. (1970) Cerebral representation of vocalization in the squirrell monkey. Exp. Brain Res. 10: 532-554
Jürgens, U., and Pratt, R. (1979) The cingular vocalization pathway in the squirrel monkey. Exp. Brain Res. 34: 499-510
Kuypers H. G. J. (1958) Corticobulbar connections to the pons and lower brain stem in man. An anatomical study. Brain 81, 364-388
Kuypers H. G. J. (1981) Anatomy of the descending pathways In: Handbook of Physiology, Section I, The Nervous System, Vol. II, Motor Systems Ed. R.E. Burke Washington American Physiological Society pp. 597-666
Price, J.L. (1996) Vocalization and the orbital and medial prefrontal cortex. In P.J. Davis and N.H. Fletcher, editors: Vocal Fold physiology; Controlling Complexity and chaos. Singular Publishing group, Inc. San Diego London ISBN 1-56593-714-7 p. 171-185
Sakuma Y., and Pfaff, D. W. (1979) Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am. J. Physiol. 237: R285-R290
VanderHorst, V. G. J. M. and Holstege, G. (1997) Estrogen induces axonal outgrowth in the nucleus retroambiguus-lumbosacral motoneuronal pathway in the adult female cat. J. Neuroscience, 17: 1122-1136
VanderHorst, V.G.J.M. and Holstege, G. (1995) Caudal medullary pathways to lumbosacral motoneuronal cell groups in the cat; evidence for direct projections possibly representing the final common pathway for lordosis. J. Comp. Neurol. 359 457-475
VanderHorst, V.G.J.M., Terasawa, E., Holstege, G. and Ralston, III, H.J (1998) Monosynaptic projections from the nucleus retroambiguus to distinct groups of lumbosacral motoneurons in the female rhesus monkey. Soc. Neurosci. Abstr. Vol. 24 Part 1. p. 950
Zhang S. P.; P. J. Davis, and R. Bandler (1995) Brain stem integration of vocalization: role of the nucleus retroambigualis. J. Neurophysiol. 74, 2500-2512
| Discussion Board | Previous Page | Your Symposium |