Invited Symposium: Pineal and its Hormone Melatonin
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The pineal gland secretes the hormone melatonin which, via binding to specific receptors in the cell membrane, conveys information about the length of the daily photoperiod to other parts of the central nervous system and peripheral tissues (Reiter, 1991). In rodents, the pineal complex consists of a superficial pineal gland, a deep pineal gland, and a stalk connecting these parts. While the superficial pineal gland has been shown to synthesise and secrete melatonin, it is still a matter of debate whether melatonin is synthesised in the deep pineal gland (Cozzi and Møller, 1988). The pineal gland consists of pinealocytes and interstitial cells. Whether the interstitial cells also contain melatonin has not been resolved. Finally, the subcellular localisation of melatonin is uncertain. Thus, some studies have indicated that melatonin, in addtion to the be located in the cytoplasm, is present also in the nuclear compartment of the cell (Mennenga et al., 1991).
We have in this study used a polyclonal antibody raised in sheep and immunohistochemistry to study the localisation of melatonin in formalin fixed cryostat section of two rodent species. Our study shows a strong immunoreactivity in both the superficial and deep pineal gland. Further, the immunoreactivity was confined to the cytoplasm and not found in the nuclear compartment. Our study indicates a secretion from the deep pineal gland to the cerebrospinal fluid of the third ventricle.
Materials and Methods
Animals:
adult male Wistar rats, weighing 250-300 g, and adult golden hamster weighing 80-110 g, were used in this investigation. The animals were kept in a 12/12 h light/darkness schedule (light on at 6 a.m.) with food and water ad libitum.
Perfusion fixation:
the animals were anaesthetised with tribromethanol and vascular perfused through the heart with ice cold heparinized (15,000 IU/l) phosphate buffered saline (pH 7.4, PBS) for 2 min followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.2) for 10 min. The brains were removed and postfixed in the same fixative for 12 h and transferred to PBS. The brains were then cryoprotected for 2 days in 20% sucrose, frozen in crushed dry ice, sectioned in a cryostat at a thickness of 15 µm and collected on gelatinized glass slides.
Immunohistochemistry:
The sections were rinsed for 3 x 5 min in PBS with 0.25% bovine serum albumin and 0.1% Triton X-100 (PBS-BT), and then incubated in a solution of 10% swine serum in PBS-BT for 30 min. They were the incubated for 24 h at 40 C with a specific antiserum against melatonin (CIDtech Res.Inc., Hamilton, Canada) raised in sheep diluted 1/1.000 in PBS-BT. This was followed by washing of the sections for 3 x 5 min in PBS-BT and incubation for 1 h with rabbit anti-goat IgG (#Z0259, DAKO, Copenhagen) diluted 1/250 in PBS-BT. After 3 x 5 min wash, the sections were incubated for 1 h in goat anti-PAP (#B0157, DAKO, Copenhagen) diluted 1/100 in PBS-BT. After a 2 x 5 min wash in PBS-BT and 10 min in 0.05 M TRIS (pH 7.6) the sections were incubated for peroxidatic activity in a solution of 0.025% diaminobenzidine and 0.003% hydrogen peroxide for 15 min. Finally, the sections were mounted on gelatinized slides, dried and embedded in DepexR.
Results
In both the hamster and rat, strong immunoreactivity was obtained in the superficial pineal gland, the deep pineal gland (Fig.1), as well as in the stalk. In all three parts the immunoreactivity was seen only in the cytoplasm of the cells (Fig.2). The nuclei never exhibited immunoreactivity (Fig.2). Staining of the perivascular spaces or the pineal capsule was never observed. It was not possible, due to the thickness of the sections, to determine whether the interstitial cells, in addition to the pinealocytes, exhibited immunoreactivity.
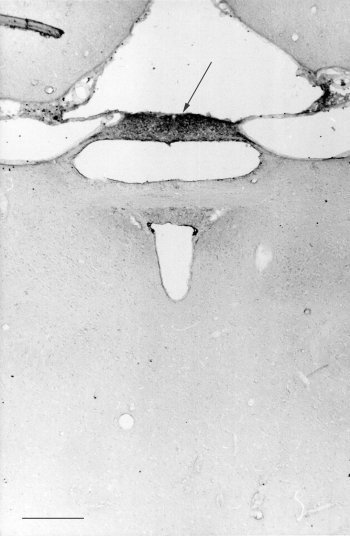 Fig. 1: photomicrograph of a frontal sections of the hamster epithalamus reacted for melatonin. Notice the strong staining of the deep pineal gland (arrows). Bar= 300 microns .
Fig. 1: photomicrograph of a frontal sections of the hamster epithalamus reacted for melatonin. Notice the strong staining of the deep pineal gland (arrows). Bar= 300 microns .
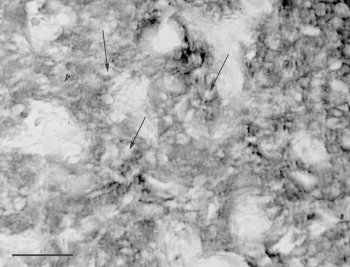 Fig. 2: photomicrograh of a part of a section of the rat superficial pineal gland reacted for melatonin. Neither the nuclei (arrows) nor the perivascular spaces are stained. Bar = 35 microns.
Fig. 2: photomicrograh of a part of a section of the rat superficial pineal gland reacted for melatonin. Neither the nuclei (arrows) nor the perivascular spaces are stained. Bar = 35 microns.
Discussion and Conclusion
Melatonin of the superficial pineal gland is secreted to the pineal venules which drain to the great cerebral vein of Galen and a high concentration of melatonin can be measured in the cerebral sinuses (Cozzi et al., 1988). However, a lower concentration of melatonin is also found in the cerebrospinal fluid (Withyachumnarnkul et al., 1980) and the hormone might in addition be secreted directly to the third ventricle from the deep pineal gland. Thus, in the hamster, the processes of pinealocytes have been shown to protrude through the ependymal lining of the pineal recess to be in contact with the cerebrospinal fluid (Welsh et al., 1989). Such a secretion is supported by the demonstration in this study of the presence of melatonin in the deep pineal gland of the hamster. Other authors (Maurizi, 1991) have suggseted melatonin to enter the cerebrospineal fluid of the lateral ventricles via a recirculation through tela choroidea.
The subcellular localization of melatonin is still not resolved. In lower vertebrates, cytological studies using radioactive labeled precursors of melatonin have indicated melatonin to be located in the dense core granules of the modified photoreceptor cells (Collin et al., 1976). These cells are phylogenetically precursor cells of the pinealocytes. However, such studies have not been successful in mammals. In addition, melatonin is a lipophilic indole and the lipid membrane of the dense core vesicle would not be able to retain melatonin inside the vesicle. This study observed immunoreativity in the cytoplasm of pineal cells but was not able to located the immunoreactivity to any cytoplasmic organelle.
Recent immunohistochemical Mennenga et al., 1991) and cell fractionation studies (Menendez-Pelaez et al., 1993) have indicated melatonin to be located also in the nucleus. In this study, such a nuclear localization could not be confirmed. However, due to the sensitivity of immunohistochemistry we would not rule out this possibility.
References
Collin JP,Calas A,Juillard MT (1976) The avian pineal organ. Distribution of exogenous indoleamines: a qualitative study of the rudimentary photoreceptor cells by electron microscopic radioautography. Experimental Brain Research 25:15-33.
Cozzi B, Møller M (1988)Indications for the presence of two popu-la-tions of serotonin-containing pin-ealocytes in the pineal com-plex of the Golden hamster (Mesocricetus auratus). An immunohistochemical study. Cell Tissue Res. 252:115-122.
Cozzi B, Ravault JP, Ferrandi B, Reiter RJ (1988) Melatonin concentration in the cerebral vascular sinuses of sheep and evidence for its episodic release. Journal of Pineal Research 5:535-43.
Maurizi CP (1991)Recirculation of cerebrospinal fluid through the tela choroidae is why high levels of melatonin can be found in the lateral ventricles. Medical Hypotheses 35:154-158.
Menendez-Pelaez A, Reiter RJ, (1993) Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. Journal of Pineal Research 15:59-69.
Mennenga K, Ueck M, Reiter RJ (1991 Immunohistological localization of melatonin in the pineal gland and retina of the rat. Journal of Pineal Research 10:159-164.
Reiter RJ (1991) Melatonin: the chemical expression of darkness. Molecular and Cellular Endocrinology 79:C153-C158.
Welsh MG. Sheridan MN, Rollag MD (1989) Cerebrospinal fluid-contacting area of the deep pineal: effects of photoperiod. Journal of Pineal Research. 7:365-380.
| Discussion Board | Previous Page | Your Symposium |