Invited Symposium: Neural Substrates of Sexual Motivation and Performance as Revealed by Neural Immediate-Early Gene Expression
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
INTRODUCTION
In recent years, our understanding of the complexity of feminine sexual behavior and its influence on reproduction has expanded, such that it is now routine to include analysis of solicitational and/or appetitive forms of behavior in our studies. In large part, this refined approach has been spearheaded by the interest in the female’s pacing behavior. Under conditions in which an estrous rat can avoid contact with males, she regulates or paces the timing of intromissions received from the male by withdrawing from the male between copulatory mounts and by reapproaching the male at given intervals to reinitiate copulation. Such pacing behavior alters the characteristics of the mating stimulation the female receives; relative to nonpaced mating, intervals between intromissions (inter-intromission interval, III) are lengthened, the duration of each intromission is longer, and the ratio of intromissions-to-mounts is increased.
Therefore, by displaying appetitive and/or solicitational patterns of behavior toward males, the female alters and controls the types, timing, and intensities of the copulatory stimulation she receives. There are major consequences of these alterations for reproduction, since paced mating selectively influences neural and behavioral function. The pacing of mating stimulation enhances the effectiveness of each intromission, so that fewer paced than nonpaced intromissions are required to induce pregnancy or pseudopregnancy [PSP] and to abbreviate the duration of estrus. Additional changes associated with paced mating are acute increases in luteinizing hormone (LH), prolactin, and 3a-androstanediol secretion and increases in extracellular dopamine levels in the nucleus accumbens and striatum.
Studies using immediate-early gene expression to delineate neural circuits involved in processing mating inputs have demonstrated that in the female, mating stimulation and artificial mechanical VCS induce an increase in the number of FOS-immunoreactive (FOS-IR) cells in several forebrain areas, including the medial preoptic area (mPOA), ventrolateral portion of the ventromedial nucleus of the hypothalamus (VMHvl), the posterodorsal division of the medial amygdala (MePD) and the posteromedial bed nucleus of the stria terminalis (BNSTpm). In these areas, FOS responses to intromissions from males or to VCS were greater than those resulting from cutaneous perineal and flank stimulation provided by mounts-without-intromission or manual palpation.
However, there is scant evidence that FOS expression has anything to do with appetitive, or motivational, components of feminine sexual behavior. Because the VMHvl is known to be critically involved in steroid-induced estrous behavior but not involved in neuroendocrine responses to mating, FOS expression in that area have been hypothesized to be associated with the performance of lordosis behavior.
However, the desire to link lordosis behavior to FOS expression is weakened by the fact that lordosis behavior is not expressed spontaneously in the absence of cutaneous and/or genitosensory stimulation from males. Hence, the question of whether there are separate brain areas activated during the expression of sexually- motivated behaviors independent of sensory stimuli originating with the male is still an open one. The experiments presented here attempted to examine whether FOS responses unique to appetitive or solicitational behaviors are expressed in the female, by examining FOS responses to paced compared to non-paced mating. Our expectation was that additional areas of FOS- immunoreactive (FOS-IR) cells might be observed over and above those activated by the genitosensory stimuli associated with intromissions, or that the same set of responsive areas would appear but that there would be alterations in the numbers of FOS-IR cells within each area.
EXPERIMENT 1: Effects of Paced Mating on FOS
In the first experiment, we examined the pattern of c-fos expression following paced vs. nonpaced ontrol treatments. As expected, the mean III was significantly longer among females receiving paced mating stimulation than among those receiving non-paced mating stimulation. Other measures of feminine sexual responsiveness, lordosis and proceptive hopping and darting behaviors, did not differ between groups.
As shown in Fig. 1, groups receiving VCS sufficient to induce PSP (5 and 15 paced intromissions and 15 non-paced intromissions) had equivalently high numbers of FOS-positive cells in the mAMYG, while groups receiving insufficient mating stimulation for PSP (5 nonpaced intromissions, mounts-only, or home cage treatments) showed significantly lower FOS. The mean number of FOS-positive cells in the 5 paced intromission group was equivalent statistically to the numbers of cells in two groups in which females received 15 intromissions, and the number of cells in the 5 non-paced intromission group was significantly lower and equivalent to the mounts- only and home cage groups. The association of the FOS response with pacing was only seen in the mAMYG; 5 paced intromissions did not significantly increase FOS responses in the MPOA, VL-VMN or BNST above mounts-only levels.
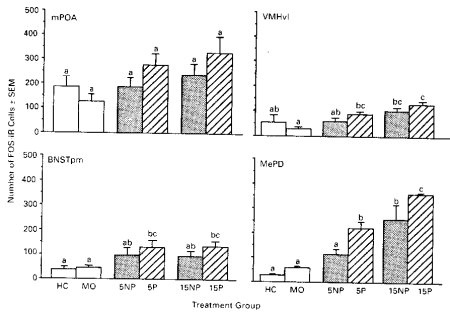 Figure 1. FOS responses to paced as opposed to non-paced mating in 4 brain areas.
Figure 1. FOS responses to paced as opposed to non-paced mating in 4 brain areas.
These results suggested that paced mating was more effective than non-paced mating in inducing FOS responses in the mmAMYG, an area which had previously been identified to respond to intromissive stimulation. However, this effect was eliminated if the numbers of intromissions were sufficiently high. Although this suggested that the sensory input rather than the display of paced mating, itself, was responsible for this effect, this finding did not rule out the possibility that the high number of intromissions represented an overload which masked the more subtle effects of motivation. Therefore, in the second experiment, we examined the patterns of FOS-IR induced under conditions in which we artificially reproduced some of the characteristics of the vaginocervical stimuli which would have occurred during paced mating, but eliminated the females’ active display of the pacing behavior, itself.
EXPERIMENT 2: FOS Responses to III and I Duration
In the second experiment, we applied stimuli which mimicked those received by the female during paced mating by experimentally prolonging the III and/or lengthening the intromission duration, two characteristics of paced mating stimulation. This was done using sexually experienced males who were placed with females at proscribed intervals in paced and non-paced mating conditions. An additional aspect of our manipulations was that, because of the manipulation, the ratio of intromissions-to-mounts was elevated as occurs in normal paced mating tests.
Our expectation was that artificially-applied stimuli might reduce the FOS expression that we had seen in the mAMYG of the 5P group in the first experiment. If this occurred, the conclusion to be drawn would be that appetitive or motivational aspects of feminine sexual behavior activate particular a population of cells, and that this group of cells, by virtue of its co-localization with FOS responses to genitosensory stimulation, could then be identified as an associated group of motivational cells within the feminine sexual behavior neural circuit.
Numbers of FOS-IR cells were quantified following mating treatments in which the III was either short (short interval, SI, approximately 40 sec) or long (long interval, LI, approximately 100-120 sec) or following mounts-without-intromission stimulation. In addition, LI females received either short (sd) or long (ld) duration intromissions from males. Therefore, groups of females received one of four mating treatments: 1) 15 mounts-without-intromission with manual separation from the male for approximately 15 sec between each mount; 2) Short interval (SI) females (n=5) were separated from the male for approximately 40 sec after each intromission before they were allowed another intromission; 3) Long interval-short duration (LIsd) females (n=5) were separated from the male for 100-120 sec, and they received intromissions of approximately 500 msec duration; 4) Long interval-long duration (LIld) females (n = 4) were separated from the male for 100-120 sec between intromissions, and they received intromissions of approximately 600 msec in duration. All fully mated females received 5 intromissions from males along with the mounts-without-intromission which occurred.
IIIs were controlled by placement and removal of a solid partition between the halves of the testing chamber for predetermined intervals.
Intromission durations were controlled by manipulating the rate at which males were able to intromit with females. Among males, a prolonged III, similar to that which occurs during paced mating, results in the expression of intromissions which are significantly longer (approximately 600 msec) than those exhibited by males who are able to intromit ad lib [approximately 500 msec; 41-43]. In this experiment, long- duration intromissions were expected when males were allowed only to intromit with the experimental female at approximately 100 sec IIIs. Males expected to show short-duration intromissions were allowed to intromit without restriction during the test and, therefore, would be expected to display short-duration intromissions. This was accomplished by the addition of an estrous stimulus female to the male's side of the test chamber whenever the experimental female was separated from the male, a procedure which essentially placed the male under non-paced mating conditions.
When manual separation of the male and female was required, only the stimulus male was handled in order to minimize possible effects of stress and handling on FOS expression in the female. Under these conditions, the sexually experienced males reinitiated copulation with the female almost immediately after withdrawal of the dividing partition. Following mating, experimental females were housed individually in plastic cages (30 X 18.5 X 12.5 cm) in the behavioral testing room until perfusion.
The results of this experiment are presented in Figures 2 and 3. As can be seen in Figure 2, there was no significant effect of experimental manipulation on the expression of FOS within the MPOA, VMHvl, and the BNSTpm. However, within the MePD, significant increases in FOS were observed when the female received the combined long interval and long duration treatments. Each of these treatments individually produced moderate but not significant increases in this area. Thus, mimicking of the type of VCS received by the female during paced mating increased the level of FOS, without the female, herself, exhibiting pacing behaviors. Exhibiting obvious signs of motivation, therefore, are not required for the increase in FOS-I cells within the MePD.
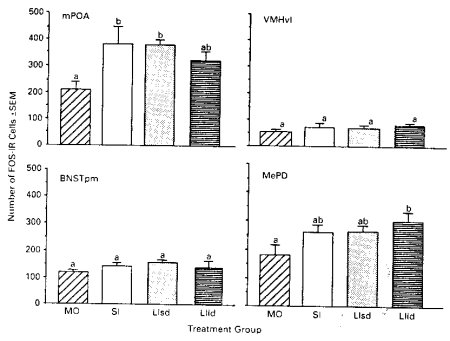
Figure 2. FOS responses in groups receiving experimentally-controlled VCS from males. Letters
which are different indicate significant differences between groups.
Outlines of he cluster of FOS-IR cells seen the MePD of the 3 mated groups are shown in Figure 3 (right) and a representative photomicrograph (left). In Figure 3A, the short-interval group is seen to have no distinct cluster in 3 of the 5 animals, and small clusters in the remaining 2 animals. By contrast, the long interval-short duration (Fig. 3B) and the long interval-long duration (Fig. 3C) groups had equivalent distinct clusters of FOS-IR cells. The mean area in µm2 of these clusters for the 3 groups were: Short-Interval: 5447±3360; Long Interval-Short Duration: 22,417±5603; Long Interval-Long Duration: 22,392±1445. Therefore, both of the Long Interval groups showed a FOS response that was not shown by the Short Interval group, suggesting that a portion of the FOS response exhibited by the females in response to paced mating is a result of prolonged III which occur under that mating condition. she receives, thereby altering the number and clustering of cells within the MePD.
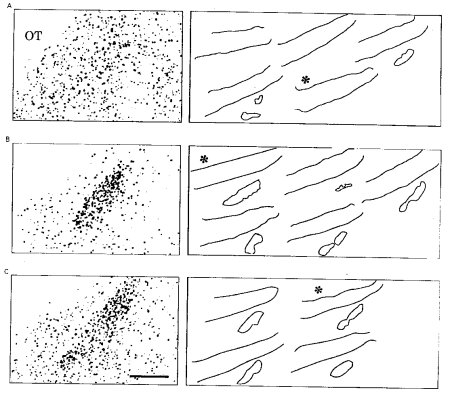 Figure 3. Asterisks indicate the outline of the section shown in the photomicrograph at left.
Figure 3. Asterisks indicate the outline of the section shown in the photomicrograph at left.
CONCLUSIONS
These results show that particular characteristics of the mating stimuli in addition to the absolute numbers of intromissions can enhance the FOS response to mating. To the extent that this enhanced response occurred in females in which the alterations in III were experimentally manipulated, this suggests that it is the particular characteristics of the VCS, itself, which stimulates cells within the MePD. However, since paced females actively prolong the III by withdrawing from the male and reapproaching him at extended intervals, the ultimate consequence of sexually motivated behavior in the female is to alter the sensory stimuli that activate cells within the MePD.
| Discussion Board | Previous Page | Your Symposium |