Invited Symposium: Neural Substrates of Sexual Motivation and Performance as Revealed by Neural Immediate-Early Gene Expression
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Fos expression following mating
Until recently, knowledge of the neural regulation of sexual behavior in male and female rodents was largely based on studying copulatory behavior following electrolytic or chemical lesions, electrical stimulation, or implantation of small quantities of steroid hormones in specific brain area (reviewed in Meisel and Sachs, 1994; Pfaff et al, 1994). However, in recent years, immunocytochemical staining of the protein products of the immediate early genes, and in particular Fos immunoreactivity (-IR), has been used to map functional neural circuits underlying copulation in the rodent brain. Immunocytochemical visualization of Fos, the protein product of the immediate early gene c-fos, has been widely used as a marker for the activation of neurons (Morgan and Curran, 1995). Mating increases Fos-IR in brain regions that have been implicated in control of sexual behavior and where, in most instances, lesions have been shown to disrupt the behavior. However, an advantage of the use of this marker is a cellular resolution that lesions cannot provide. As a result of this greater resolution, activated neurons were observed in discrete subdivisions within the brain areas involved in sexual behavior. Moreover, differences in distribution of Fos-IR neurons or differences in numbers of activated neurons could be correlated with the different elements of sexual behavior. The present paper focuses on studies in the male rat. However, striking similarities exist between rats and other rodents, as well as between males and females. For a better understanding of these similarities, but also differences, see other papers presented in this symposium.
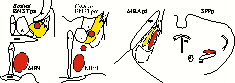
In male rats (Baum and Everitt, 1992; Veening and Coolen, 1998), but also in male hamsters (Newman et al, 1997) and gerbils (Heeb and Yahr, 1996), copulation induces Fos-IR in subdivisions of the medial preoptic area, bed nucleus of the stria terminalis (BNST), medial amygdala (MEA), and the posterior thalamus (or central tegmental field). Induction of Fos-IR is most dramatic in the medial preoptic nucleus (MPN), posteromedial part of the BNST (BNSTpm), posterodorsal part of the MEA (MEApd), and parvocellular subparafascicular thalamic nucleus (SPFp; Coolen et al, 1996,1997a). These brain regions act in concert to regulate male sexual behavior. However, male sexual behavior is not just one behavior. Instead it is a complex of different behaviors, including appetitive behaviors, such as chemosensory or anogenital investigation of the female, and consummatory behaviors, i.e. mounting, intromissions and ejaculations. Moreover, comparison between induction of Fos-IR following anogenital investigation of a female versus copulation that included ejaculation, revealed differences in the numbers of Fos-IR neurons and in the distribution of activated neurons, in the brain regions mentioned above (Coolen et al, 1997a). These results are summarized in Figure 1, which schematically illustrates distributions of Fos-IR neurons in coronal sections. In particular, anogenital investigation activated Fos-IR neurons in BNSTpm, and in a medial portion of MEApd (illustrated in yellow), thus suggesting that these brain regions are involved in display of chemosensory investigation. Copulation that included ejaculation resulted in higher numbers of Fos-IR neurons in these areas, and in a specific pattern of activation (illustrated in red). In particular, copulation, but not anogenital investigation was followed by activation in MPN and SPFp, as well as in a lateral portion of MEApd, and two distinct clusters in rostral and caudal BNSTpm. This suggests that these areas were mainly involved in the performance of mating.
 Click to enlarge
Fig. 1: Neural activation following anogenital investigation (yellow) versus copulation (red).
Click to enlarge
Fig. 1: Neural activation following anogenital investigation (yellow) versus copulation (red).
Ejaculation-subcircuit
The previous section described the differences in distribution of neural activation following copulation compared to anogenital investigation. However, the distribution of Fos-IR was studied without taking into account the various elements of mating (i.e. mounts, intromissions, or ejaculation). Therefore, it was unclear which behavioral elements specifically resulted in the copulation-induced neural activation in the brain regions illustrated in Figure 1. Two separate studies from our laboratory indicated that the patterns of Fos-IR observed following copulation, are specifically related to ejaculation. First, neural activation was studied in males that displayed mounts but no intromissions; in males that displayed intromissions and mounts but no ejaculation; and finally in males that displayed ejaculation, intromissions, and mounts (Coolen et al, 1996). The results from this study indicated that the pattern of neural activation that included clusters of Fos-IR in lateral MEApd, rostral and caudal BNSTpm, and SPFp, was specific to ejaculation, and activation was not induced by intromissions or mounts. In contrast, the MPN is involved in all consummatory elements of copulation and increasing Fos-IR was induced by increasing sexual activity.
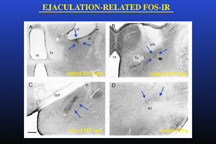
However, since Fos-IR was studied following full mating, it could not be determined whether this specific pattern of Fos-IR was specifically related to ejaculation itself or instead reflected a summation of sexual activity including ejaculation but also intromissions and mounts that precede the ejaculation. To test the hypothesis that the Fos-IR neural clusters present after ejaculation are related to ejaculation per se, neural activation following mating was studied in males that displayed ejaculation with minimal preceding sexual activity (Coolen et al, 1997b). To accomplish this, male rats received systemic injections of the serotonergic 5-HT1A receptor agonist 8-OH-DPAT, which has strong facilitatory effects on male sexual behavior. Administration of 8-OH-DPAT resulted in low numbers of mounts and intromissions preceding ejaculation, and a short ejaculation latency. Nonetheless, mating in 8-OH-DPAT-treated males resulted in a similar clusters of neural activation and similar numbers of Fos-IR neurons, as in control "normal" mated, saline-treated males. Moreover, in males that ejaculated with the first mount, without any preceding sexual activity, similar clusters of Fos-IR were present. Administration of the drug by itself in non-mating control males did not increase Fos-IR in the brain areas where ejaculation-related Fos-IR was observed. Together with our previous finding that intromissions alone do not induce the presence of the characteristic clusters of Fos-IR neurons, these results support the existence of a specific ejaculation-related subcircuit within a larger circuit involved in male sexual behavior. Figure 2 illustrates the Fos-IR clusters observed following ejaculation in male rats.
 Click to enlarge
Fig. 2: Ejaculation-related neural activation.
Click to enlarge
Fig. 2: Ejaculation-related neural activation.
The precise function of the ejaculation-related neuronal clusters remains to be determined. A common question in studies using Fos as a marker for activation is whether the Fos expression is related to sensory inputs, behavioral output, or both. Neural activation in the ejaculation-related clusters appears to be related to genital sensory inputs. In female rats, similar Fos-IR clusters are present in the same subregions following vaginocervical stimulation (Coolen et al, 1996). Thus, it is unlikely that is neural activation in males is being triggered by display of ejaculatory behavior. Likewise, in rats and hamsters, lesions in each of the subnuclei containing the ejaculation-related clusters separately do not completely abolish display of ejaculatory behavior, indicating that the individual clusters are not required for triggering ejaculation. Interestingly, studies in hamsters have indicated a role for the ejaculation-related neural activity in the lateral MEApd in sexual satiety (Parfitt et al, 1998). For more information, see the paper presented by D. Parfitt in this symposium.
Relay of sensory inputs to the MPN
In the previous sections it was demonstrated that the use of Fos expression as a marker for neural activation has provided a better understanding of the circuits underlying male sexual behavior at a cellular level. A major advantage of this cellular resolution is the possibility to characterize the connections and neurochemical phenotype of these neurons. A large portion of my work has focused on the question whether the Fos-activated neurons are involved in relay of sensory information. The MPN, BNSTpm, MEApd, and SPFp form a heavily interconnected network through which sensory information can be relayed. The MPN is an essential site for the regulation of male copulation and appears to be in an unique position to integrate sensory and hormonal information associated with the control of male copulation. It can receive olfactory sensory inputs through MEA and BNST, both of which have connections with the olfactory bulbs, and show Fos-activation following chemosensory inputs (section1; Coolen et al, 1997a; Bressler and Baum, 1996). In addition, genital sensory inputs can be relayed to MPN via the SPFp, which receives input from spinal cord, and where Fos is present following genital sensory inputs (sections 1 and 2).
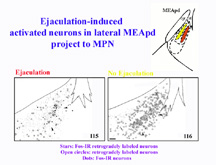
To determine whether the Fos-IR neurons in BNSTpm, MEApd, and SPFp, are involved in the relay of sensory inputs to the MPN, mating-induced Fos-IR was combined with anterograde and retrograde tract tracing in the MPN (Coolen et al, 1998). The results from this study demonstrated that Fos-IR neurons in the ejaculation-related clusters in MEApd, BNSTpm, and SPFp, are involved in bidirectional connections with MPN. Following retrograde tracer injections, copulation induced Fos-IR in retrogradely labeled neurons in BNSTpm, MEApd, and SPFp. Interestingly, activated neurons that project to MPN were present only in the clusters where Fos-IR is related to ejaculation. Furthermore, following mating that did not include ejaculation, no Fos-IR retrogradely labeled neurons were detected. Therefore, this suggests that ejaculation-induced Fos-IR neurons are involved in projections to MPN, possibly relaying ejaculation-specific, or genital sensory inputs. As an example, Figure 3 illustrates the correlation of the distributions of retrogradely labeled neurons in MEApd that project to MPN, and mating-induced Fos-IR. The stars indicate the location of activated neurons that project to MPN, while the open circles are neurons that project to MPN, but are not activated following mating. The panel on the left shows a male that mated to ejaculation, while the right panel shows the lack of double-labeling following mating that did not include ejaculation.
 Click to enlarge
Fig. 3: Ejaculation-induced Fos-IR neurons in MEApd project to MPN.
Click to enlarge
Fig. 3: Ejaculation-induced Fos-IR neurons in MEApd project to MPN.
The findings of this study suggested that neurons in BNSTpm and MEApd that express Fos following chemosensory stimulation are not involved in the direct relay of chemosensory information to the MPN. This was a surprising finding, since these brain regions are thought to be involved in the relay of chemosensory inputs. It is possible that interneurons are involved in relay of chemosensory inputs and that the entire chain of neurons does not express Fos. However, it is also possible that Fos expression following mating is not related specifically to chemosensory cues. Therefore, further studies are needed to address this question.
In addition to providing inputs to MPN, ejaculation-related neural clusters also receive input from MPN. Following anterograde tracer injections in MPN, anterogradely labeled fibers were observed closely surrounding Fos-IR neurons in these brain regions. Moreover, the projections arising from the MPN terminate densely in the small subdivisions where neural activation is related to ejaculation. Taken together with previous findings (section 2), this again emphasizes the existence of a subcircuit that expresses neural activation after ejaculation within the larger network underlying male sexual behavior.
SPFp: Relay of genital sensory inputs
The studies described in the previous sections indicate the involvement of a portion of the activated neurons in relay of genital sensory inputs. However, the pathway through which genital sensory cues are relayed is still unclear. The SPFp is in a position to relay genital sensory cues to other brain regions involved in male sexual behavior. The posterior thalamus that includes SPFp, receives projections from regions of the spinal cord that are most relevant for copulation, i.e. the lumbosacral spinal cord. In addition, activated neurons in SPFp project to the MPN. Yet, it is unknown which neurons in the lumbosacral spinal cord provide input to SPFp. In addition, it is unknown if SPFp projects to regions of BNSTpm and MEApd, where mating-induced neural activation is present.
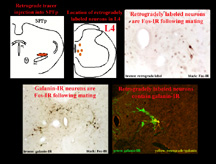
Preliminary work from our laboratory has addressed these questions (Coolen et al, 1997c; Veening et al, 1997). With the use of retrograde tracer injections into SPFp, the pathway through which SPFp receives input from the spinal cord was identified. This pathway consists of neurons in lumbosacral spinal cord that are located in lamina X of L3, L4, and L5, and contain the neuropeptides galanin and cholecystokinin (CCK). Moreover, these neurons are activated following ejaculation, but not following intromissions alone. In turn, these neurons project to a medial portion of SPFp, where galanin-IR and CCK-IR fibers terminate and closely surround ejaculation-induced Fos-IR neurons. This pathway is illustrated in Figure 4. Figure 4 shows neurons in a coronal section through lumbosacral spinal cord that are retrogradely labeled after retrograde tracer injections in the medial portion of SPFp.
 Click to enlarge
Fig. 4: Spino-thalamo pathway.
Click to enlarge
Fig. 4: Spino-thalamo pathway.
With the use of anterograde tracer injections in SPFp the projections to MEApd, BNSTpm, and MPN were investigated. Preliminary results indicate that the projections of the medial portion of SPFp that receives inputs from activated neurons in lumbosacral spinal cord, closely correlate with the distribution of mating-induced Fos-IR neurons. Figure 5 illustrates the correlation of efferent fibers from SPFp and mating-induced Fos-IR neurons in the MEApd.
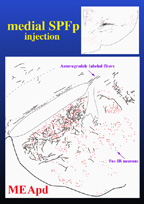 Click to enlarge
Fig. 5: Projections from SPFp to activated neurons in MEApd.
Click to enlarge
Fig. 5: Projections from SPFp to activated neurons in MEApd.
Together these results indicate that the SPFp is in the position to receive genital sensory inputs from lumbosacral spinal cord, and to relay these inputs to other brain regions involved in male sexual behavior.
In conclusion, the use of Fos as a marker for neural activation has been a powerful tool in studying the neural circuits that underly male sexual behavior. Not only does the use of this marker provide an anatomical detail and cellular resolution that cannot provided by other techniques, but this approach also provides the possibility to characterize the connections and neurochemical content of these neurons, and to study their role in the relay of sensory, hormonal, or other information.
References
- Baum, M.J., and B.J. Everitt BJ (1992) Increased expression of c-fos in the medial preoptic area after mating in male rats: Role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neurosci. 50:627-646.
- Bressler, S.C., and M.J. Baum (1996) Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neurosci. 71:1063-1072.
- Coolen, L.M., H.J.P.W., Peters, and J.G. Veening (1996) Fos-immunoreactivity in the rat brain following consummatory elements of sexual behavior: A sex comparison. Brain Res. 738:67-82.
- Coolen, L.M., H.J.P.W Peters, and J.G. Veening (1997a) Distribution of Fos-immunoreactivity following mating versus anogenital investigation in the male rat. Neurosci. 77:1151-1161.
- Coolen, L.M., H.J.P.W Peters, and J.G. Veening (1998) Anatomical relationships of the medial preoptic area and other brain regions activated following male sexual behavior: A commbined Fos and tract-tracing study. J. Comp. Neurol. 397: 421-435.
- Coolen, L.M., B. Olivier, H.J.P.W. Peters, and J.G. Veening (1997b) Demonstration of ejaculation-induced neural activity in the male rat brain using 5-HT1A agonist 8-OH-DPAT. Physiol. Behav. 62:881-891.
- Coolen, L.M., J.G. Veening, A.Z. Murphy, and M.T. Shipley (1997c) Projections of subparafascicular nucleus: Evidence for a discrete spino-thalamo-forebrain circuit activated by mating. Soc. Neurosci. Abstr. 532.2.
- Heeb, H.M., and P. Yahr (1996) C-fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neurosci. 72:1049-1071.
- Morgan, J.I., and T.E. Curran (1995) Proto-oncogenes. Beyond second messengers. In Psychopharmacology: The fourth generation of progress. (Eds. Bloom and Kupfer) pp.631-642. Raven Press, New York.
- Meisel, R.L., and B.D. Sachs (1994) The physiology of male sexual behavior. In Knobil, E., and J.D. Neill (eds): The physiology of reproduction, second edition. New York: Raven Press, pp 3-105.
- Newman, S.W., D.B. Parfitt, and S. Kollack-Walker (1997) Mating-induced c-fos expression patterns complement and supplement observations after lesions in the male syrian hamster brain. Ann. NY Acad. Sci. 807: 239-259.
- Parfitt, D.B., L.M. Coolen, S.W. Newman, and R.I. Wood (1998) Lesions of the posterior medial nucleus of the amygdala delay sexual satiety. Submitted to Behav. Brain Res.
- Pfaff D.W., S. Schwartz-Giblin, M.M. McCarthy, and L.-M. Kow (1994) Cellular and molecular mechanisms of female reproductive behaviors. In Knobil, E., and J.D. Neill (eds): The physiology of reproduction, second edition. New York: Raven Press, pp 107-220.
- Veening, J.G., and L.M. Coolen (1998) Neural activation following sexual behavior in the male and female brain. Behav. Brain. Res. 92:181-193.
- Veening J.G., Coolen L.M., Murphy A.Z. and Shipley M.T. (1997) Ejaculation, but not pelvic nerve stimulation, activates galanin-IR pathways between lumbosacral spinal cord and subparafascicular thalamic nucleus, in the male rat. Soc. Neurosci. Abstr. 532.1.
| Discussion Board | Previous Page | Your Symposium |