Poster
Contents
Hypoxic-ischemic
encephalopathy
| INABIS '98 Home Page | Your Session | Related Symposia & Posters | Scientific Program | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
In order to study the role of free radicals in oxidative injury, we developed several methods that allow us to measure different aspects of the processes associated with the production of reactive oxygen species and the resulting injury. The hydroxyl radical is a free radical species that is considered to be extremely reactive and is among the most toxic. Increased production of hydroxyl radicals leads to membrane lipid peroxidation, protein aggregation and DNA hydroxylation. Hydroxyl radicals will in fact react with most biomolecules and cause injury. A better understanding of the disturbances in homeostatic regulatory mechanisms associated with such injury are made possible by the availability of methods described below.
Hydroxyl radicals
Free radicals have very short half-lives and are difficult to measure. The extremely reactive hydroxyl radical has an estimated half-life of 10-9 sec., hence its in-vivo detection requires trapping methods. Aromatic compounds such as salicylate, 3- or 4-hydroxybenzoate, aminosalicylate, tyrosine or phenylalanine undergo addition reactions with hydroxyl radicals producing characteristic, stable products of hydroxylation. Our laboratory developed a gas chromatography/mass spectrometry (GC/MS) method to detect the production of hydroxyl radicals using salicylate as a probe (1). Salicylate is safe to administer to humans, it reaches concentrations in body fluids sufficient to scavenge hydroxyl radicals, the hydroxylation products are stable and are present at measurable levels even under control conditions, and their concentration is proportional to the hydroxyl radicals formed in-vivo. Salicylate hydroxylation products and related aromatic compounds were extracted and analyzed as trimethylsilyl derivatives by GC/MS. Quantitation was achieved by selected-ion-recording (SIR) with benzoic acid (ring-D5) as an internal standard. Standard curves were linear in the concentration ranges investigated (0.1-10 nmol) for all individual compounds. Recovery from human plasma was in the range of 90-102%. The detection limit was between 50 fmol and 1 pmol per 1 µl injection. The within-run and between-run coefficients of variation were between 4.6-9.1%. We were able to detect the baseline levels of hydroxylation products in human fibroblasts after incubation with salicylate.
Toxic aldehydes
The major products of lipid peroxidation are saturated and unsaturated aldehydes. The extremely broad spectrum of biological effects of aldehydic lipid peroxidation products has necessitated the development of a technique that can quantitate all of the aldehydes formed in biological materials. The method developed in our laboratory (2) is based on the use of O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride (PFBHA.HCl) to form the O-pentafluorobenzyl-Oxime (PFB-Oxime) derivatives of 22 saturated and unsaturated aldehydes (C2-C12) including hexanal, 4-hydroxy-non-2-enal (HNE) and malondialdehyde (MDA), followed by silylation (TMS) of the hydroxyl group to trimethylsilyl ethers. The PFB-Oxime-TMS derivatives were analyzed by GC/MS with negative ion chemical ionization. Quantitation was achieved using benzaldehyde (ring-D5) as an internal standard in selected ion recording (SIR) mode. Standard curves were linear (r>0.99) for all individual aldehydes. The detection limit was between 50 fmol and 1 pmol per 1 µl injected aldehyde. The coefficient of variability (CV) for within-run and between-run assay precision for measuring aldehydes was assessed using 10 replicate samples of 5 separate experiments, and was found to be between 5-10%. A representative standard curve for hexanal is shown in figure 1. The high sensitivity of this method allows the measurement of physiological aldehyde levels in biological samples.
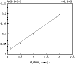 Figure 1: Standard curve for hexanal.
Figure 1: Standard curve for hexanal.
Acyloins
Aldehydes are relatively more stable than free radicals, and thus are able to diffuse in the body and accumulate in tissues. Saturated and unsaturated aldehydes are very reactive towards molecules containing sulfhydryl (-SH) groups. Some compounds that contain this functional group include cysteine, glutathione, coenzyme A, and many proteins and enzymes. The inactivation of these molecules leads to cellular injury. Therefore, the enzymatic metabolism of aldehydes to less toxic substances is an important aspect of protection against cell damage. Studies have shown that pyruvate dehydrogenase (PDH) catalyzes a little known conjugation reaction between pyruvate and saturated aldehydes to produce acyloins (3-hydroxyalkan-2-one) (Figure 2), which can then be reduced endogenously to the corresponding 2,3-alkanedials. Since acyloins and 2,3-alkanedials are stable and less toxic products, these reactions may serve as an alternative and ubiquitous detoxification mechanism for saturated aldehydes produced by lipid peroxidation. Various kinetic aspects of this enzymatic reaction were studied in-vitro by incubating rat heart homogenate in a buffer solution containing hexanal, pyruvate and other cofactors. The products formed were then prepared for and measured by the same GC/MS method we used for the analysis of aldehydes (2).
Acyloin production versus time by rat heart homogenates was assayed concurrently with the disappearance of hexanal (see Figure 3). Acyloin production was quantitated by equating the disappearance of hexanal with the appearance of 3-hydroxyocta-2-one (3-OHO) (Figure 4). The stoichiometry of this reaction is such that the disappearance of 1 mole of hexanal corresponds to the appearance of 1 mole of 3-OHO. The standard curve for 3-OHO was based on the amount of measurable free hexanal.
The results of our in-vitro studies showed a decrease in aldehyde concentration and an increase in acyloin concentration over time (Figure 5). These initial results strongly suggest that the metabolism of hexanal to 3-OHO by PDH may be an important reaction in the removal and detoxification of toxic aldehydes.
![]() Figure 2: Mechanism of PDH-catalyzed formation of 3-OHO from
hexanal.
Figure 2: Mechanism of PDH-catalyzed formation of 3-OHO from
hexanal.
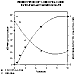 Figure 3: Production of 3-OHO from hexanal versus time.
Figure 3: Production of 3-OHO from hexanal versus time.
 Figure 4: Hexanal-dependent formation of 3-OHO by rat heart
homogenate.
Figure 4: Hexanal-dependent formation of 3-OHO by rat heart
homogenate.
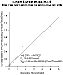 Figure 5: Lineweaver-Burk plot for 3-OHO production from hexanal.
Figure 5: Lineweaver-Burk plot for 3-OHO production from hexanal.
Role of free radicals in DOX-induced cardiotoxicity
The anthracycline antibiotic, doxorubicin (DOX, also known as adriamycin) is a potent broad spectrum chemotherapeutic agent that is effective against human malignancies such as leukemias, lymphomas and many solid tumors and is widely used in both pediatric and adult patients. DOX produces a number of side effects. The acute effects are usually transient and clinically manageable. The long-term effects include the insidious onset of cardiomyopathy that often leads to congestive heart failure. The incidence of cardiotoxicity increases at cumulative doses in excess of 500 mg/m2 and is age related. Younger children are at higher risk and DOX treatment in children may lead to a lifelong reduction in myocardial mass which may result in decreased cardiac reserve in later life. Although the precise mechanism of DOX-induced cardiac injury is not clear, drug-stimulated reactive oxygen metabolism that causes peroxidation of membrane lipids is thought to be most likely factor responsible for the development of cardiotoxicity.
Using in-vivo rat models in which DOX is given either as a single bolus injection or over a two week period in divided doses, we investigated the DOX-induced cascade of early and long-term biochemical changes. The experiments focus on the role of toxic aldehydes that initiate the process ultimately leading to cardiomyopathy. We also tested the effects of L-carnitine, a non-toxic agent that has been found to protect against DOX-induced cardiac toxicity in an animal model.
Animals and drug treatment
Acute experiments: Male Wistar rats (Charles River, Montreal, Canada, 400-450g) were treated with a bolus injection of 10 mg/kg of DOX (Adria Laboratories, Columbus, OH) in 0.9% NaCl solution intraperitoneally, and were sacrificed at 1h, 2h, 4h, 8h and 24h after DOX injection.
Chronic experiments: A total of 30 male Wistar rats were divided into six groups with 5 rats in each group. Group A (control) received daily intraperitoneal injections of 0.5 ml 0.9% NaCl for 5 wks. DOX was administered intraperitoneally to rats in six equal doses of 2.5 mg/kg each over a two week period. Group B received the full schedule of DOX (2 wks) and daily intraperitoneal 0.5 ml 0.9% NaCl injections (5 wks); Group C received daily intraperitoneal L-carnitine injections (500 mg/kg in 0.5 ml 0.9% NaCl) for 5 wks; Group D received the full schedule of DOX (2 wks) and daily intraperitoneal L-carnitine for 5 wks; Group E received the full schedule of DOX (2 wks) and daily 0.5 ml 0.9% NaCl injections (2 wks); Group F received only a single dose of DOX (2.5 mg/kg). Rats in group A, B, C and D were sacrificed at the end of week 5. Rats in group E and F were sacrificed 2 h after the last dose or the single dose of DOX.
Experimental methods
Toxic aldehydes and acyloins were measured as described above by a method developed in our laboratory (2). Cardiac functional abnormalities following DOX treatment were documented by echocardiography. Myosin Light Chain-1 (MLC-1) was measured in rat plasma by an ELISA assay from Spectral Diagnostics, Inc. (Toronto, Ontario, Canada).
Results
Acute effects of DOX treatment (3): Aldehyde and acyloin concentrations were measured in rat plasma (A) and heart (B) at various times after 10 mg/kg of bolus injection of DOX (Figure 6). Total aldehydes in rat plasma and heart tissue increased significantly, and peaked at 2 hours following in-vivo DOX treatment. The aldehyde levels returned to control values 8-24 h after DOX administration. Figure 7 shows the changes of acyloins in rat plasma (A) and heart (B) after DOX treatment. Total acyloin levels in rat plasma increased at 1 h after DOX treatment, and then significantly decreased. There was a significant decrease of acyloins in heart tissue following DOX treatment. The simultaneous increase in aldehydes and decrease in acyloins indicate that DOX not only causes increases in free radical and aldehyde production, but also appears to inhibit the PDH-catalyzed detoxification of toxic aldehydes.
![]() Figure 6: Total aldehydes in rat plasma and heart. Data presented
as mean ± SD.
* p<0.01.
Figure 6: Total aldehydes in rat plasma and heart. Data presented
as mean ± SD.
* p<0.01.
![]() Figure 7: Total acyloins in rat plasma and heart. Data presented as
mean ± SD.
* p<0.01,
# p<0.05.
Figure 7: Total acyloins in rat plasma and heart. Data presented as
mean ± SD.
* p<0.01,
# p<0.05.
Chronic effects following repeated DOX treatment (4): Concentrations of total aldehydes and some of the most toxic individual aldehydes in rat plasma and heart are shown in figures 8 and 9. Total aldehydes significantly increased 2 h after a single, 2.5 mg/kg dose of DOX. Rats in group E, in which each animal received the full 15 mg/kg of DOX schedule and the samples were taken 2 h after the last dose, had the highest aldehyde concentrations. In group B, aldehyde concentrations remained significantly higher than control even 3 weeks after the final dose of DOX. In group D, in which DOX was given together with L-carnitine, aldehyde levels were significantly lower than in group B. Although aldehyde levels in heart tissue remained higher than control, there was no difference in plasma aldehydes between group D and control. Individual toxic aldehydes (hexanal, HNE and MDA) revealed similar patterns.
MLC-1, a marker of cardiac injury, was measured in rat plasma in all groups of experimental animals as shown in Figure 10. Groups B, C and D were the statistically indistinguishable from control, while group F and E were significantly elevated. MLC-1 was higher in group E than in group F, indicating a greater injury to the heart following repeat injections of DOX than after a single dose.
Echocardiographic measurements of cardiac function did not reveal any statistically significant differences between control and any of the treatment groups.
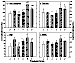 Figure 8: Total and selected individual toxic aldehyde concentrations
in rat plasma. Data presented as mean ± SD (nmol/L).
* p<0.05,
** p<0.01, versus Group A;
# p<0.05,
## p<0.01, versus Group B;
+ p<0.05,
++ p<0.01, Group D versus Group C;
& p<0.05,
&& p<0.01, Group F versus Group E.
Figure 8: Total and selected individual toxic aldehyde concentrations
in rat plasma. Data presented as mean ± SD (nmol/L).
* p<0.05,
** p<0.01, versus Group A;
# p<0.05,
## p<0.01, versus Group B;
+ p<0.05,
++ p<0.01, Group D versus Group C;
& p<0.05,
&& p<0.01, Group F versus Group E.
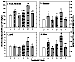 Figure 9: Total and selected individual toxic aldehyde concentrations
in rat heart. Data presented as mean ± SD (pmol/100 mg wet weight).
* p<0.05,
** p<0.01, versus Group A;
# p<0.05,
## p<0.01, versus Group B;
+ p<0.05,
++ p<0.01, Group D versus Group C;
& p<0.05,
&& p<0.01, Group F versus Group E.
Figure 9: Total and selected individual toxic aldehyde concentrations
in rat heart. Data presented as mean ± SD (pmol/100 mg wet weight).
* p<0.05,
** p<0.01, versus Group A;
# p<0.05,
## p<0.01, versus Group B;
+ p<0.05,
++ p<0.01, Group D versus Group C;
& p<0.05,
&& p<0.01, Group F versus Group E.
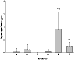 Figure 10: Myosin Light Chain-1 in rat plasma following DOX and
L-carnitine treatment. Data presented as mean ± SE,
* P<0.05, Group A compared to Group F and E,
# P<0.05 Group E versus Group F.
Figure 10: Myosin Light Chain-1 in rat plasma following DOX and
L-carnitine treatment. Data presented as mean ± SE,
* P<0.05, Group A compared to Group F and E,
# P<0.05 Group E versus Group F.
Discussion
The results indicate that free radical-mediated lipid peroxidation occurs early after DOX administration, and aldehyde concentrations are a very sensitive parameter to monitor this injurious process. The data also revealed that aldehyde production occurs in a cumulative fashion, and might parallel the dose-dependent and cumulative risk of DOX-induced cardiotoxicity. The changes included increases of some of the most toxic aldehydes such as hexanal, HNE and MDA, which may be important mediators of DOX-induced cardiac toxicity. These results suggest that multiple doses of DOX cause a progressive impairment of the antioxidant reserves of the heart, an organ that has less antioxidant capacity and appears to be much more vulnerable to DOX toxicity than other tissues. Our data also provided direct evidence that exogenous L-carnitine can attenuate the extent of doxorubicin-induced lipid peroxidation.
Our results further indicate that injury to the myocardium as measured by release of MLC-1 is greater after six doses that after a single dose, even if measured at the same time (2 h) after the last treatment. This supports our data with aldehydes that repeat exposure to DOX causes cumulative injury, and suggests that antioxidant protective mechanisms in the heart are less able to prevent damage after repeat exposure than after a single treatment.
A comparison of the three methods used in this study to monitor the toxic effects of chronic DOX treatment indicates that echocardiographic measures of cardiac function and biochemical changes do not parallel each other. Echocardiographic changes were not sensitive enough to detect injury in this model. In contrast, MLC-1 levels rose early following DOX treatment and appear to be a sensitive and early marker of injury as it is occurring. While both cardiac markers and lipid peroxidation products rose following DOX exposure, aldehyde elevations persisted much longer. The persistently high aldehyde levels three weeks after the last exposure to DOX suggest that aldehyde measurement might be more sensitive not only as a marker of injury, but also as a reflection of a change in the balance between production of reactive oxygen species (ROS) and protection by antioxidants, indicating increased susceptibility to future injury.
DOX induced cardiac toxicity is a free radical-mediated lipid peroxidation process. The resulting injury occurs in a cumulative fashion, and is probably due to a progressive impairment of the antioxidant defenses of the heart. The persistent elevations of aldehydes appear to parallel the dose-dependent and cumulative characteristics of DOX-induced cardiotoxicity.
Free radicals and Complex I deficiency
The production of ATP by ATP Synthase (Complex V) is an essential product of oxidative phosphorylation. This process involves transfer of electrons from NADH to O2 by Complexes I-IV of the mitochondrial respiratory chain. Inherited defects in any of these complexes lead to reduced energy production and disease. Previous studies suggested the involvement of oxygen free radicals in the etiology of cardiomyopathy with cataracts. To investigate the role of free radicals in the pathogenesis of the cardiomyopathy with cataracts and complex I deficiency, fibroblasts from patients were assessed for hydroxyl radical formation and aldehydic lipid peroxidation products with and without redox active agents that increase free radicals (5).
Patients
Patient 5221 was a female infant born at 42 weeks. She showed right ventricular hypertrophy, hypertrophic cardiomyopathy, poor ventricular function and had bilateral cataracts. She died at 12 days of age due to cardiac failure. Patients 5624 and 6275 were from two different families who had Complex I deficiency. They both presented with hepatopathy and tubulopathy, but without cardiomyopathy or cataracts. Patient 5624 died from neurodegeneration (Leigh’s Disease), while patient 6275 is still alive without neurodegeneration.
Skin fibroblasts culture
Human skin fibroblasts (Cell lines: Control 4212; Patient 5221; Patient 5624; Patient 6275) were grown from explants of forearm skin biopsies and cultured in eagles-minimal essential medium (-MEM) supplemented with 10% (v/v) fetal calf serum and 10.5 mM glucose. Biochemical measurements of hydroxyl radicals, aldehydes and acyloins were performed as described above in several normal fibroblast cell lines as well as in the cells of affected patients. The data shown in the Results section for control is from 4212, a representative control cell line.
Results
The rate of hydroxyl radical formation in patient cells was increased 2 to 10-fold under basal conditions, and up to 20-fold following menadione or doxorubicin treatment compared to normal cells (Fig 11). We also found an overproduction of aldehydes in patient cells both under basal conditions, and after treatment. Both hydroxyl radicals and toxic aldehydes such as hexanal, 4-hydroxynon-2-enal, and malondialdehyde were elevated in cells from patients with three types of Complex I deficiency (Fig 12). In contrast, acyloins, the less toxic conjugated products of pyruvate and saturated aldehydes, were lower in the patient cells (Fig 13).
Our data provided direct evidence for the first time that Complex I deficiency is associated with:
- excessive production of hydroxyl radicals and lipid peroxidation
- inhibition of the removal of toxic aldehydes via conversion to less toxic acyloins.
This shift in the balance between production and removal of ROS in favor of oxygen free radicals will lead to damage which may contribute to the early onset of cardiomyopathy and cataracts, and death in early infancy in patients affected with this disease.
 Figure 11: Products of hydroxyl radical attack on salicylate in
fibroblasts. Cells were treated with 2 µM salicylate and 25 µM
menadione or doxorubicin for 2 h. Data presented as mean ± SD. (cell line
4212, control; cell line 5221, Complex I deficiency patient 5221 with cardiomyopathy
and cataracts; cell lines 5624 and 6275, Complex I deficiency patient 5624 and 6275
with hepatopathy and tubulopathy.
* p<0.05,
** p<0.01 compared to control cell;
# p<0.05,
## p<0.01 compared to cell line 5221.)
Figure 11: Products of hydroxyl radical attack on salicylate in
fibroblasts. Cells were treated with 2 µM salicylate and 25 µM
menadione or doxorubicin for 2 h. Data presented as mean ± SD. (cell line
4212, control; cell line 5221, Complex I deficiency patient 5221 with cardiomyopathy
and cataracts; cell lines 5624 and 6275, Complex I deficiency patient 5624 and 6275
with hepatopathy and tubulopathy.
* p<0.05,
** p<0.01 compared to control cell;
# p<0.05,
## p<0.01 compared to cell line 5221.)
 Figure 12: Aldehyde and acyloin production in different cells after
menadione treatment. Cells were treated with 25 µM menadione for 2 h. Data
presented as mean ± SD. (cell line 4212, control; cell line 5221, Complex
I deficiency patient 5221 with cardiomyopathy and cataracts; cell lines 5624 and
6275, Complex I deficiency patient 5624 and 6275 with hepatopathy and tubulopathy.
* p<0.05,
** p<0.01 compared to control cell;
# p<0.05,
## p<0.01 compared to cell line 5221.)
Figure 12: Aldehyde and acyloin production in different cells after
menadione treatment. Cells were treated with 25 µM menadione for 2 h. Data
presented as mean ± SD. (cell line 4212, control; cell line 5221, Complex
I deficiency patient 5221 with cardiomyopathy and cataracts; cell lines 5624 and
6275, Complex I deficiency patient 5624 and 6275 with hepatopathy and tubulopathy.
* p<0.05,
** p<0.01 compared to control cell;
# p<0.05,
## p<0.01 compared to cell line 5221.)
 Figure 13: Aldehyde and acyloin production following increasing doses
of menadione or doxorubicin. Cells were treated with menadione (10, 25 and 50
µM) or doxorubicin (5, 25 and 50 µM) for 2 h. Data presented as mean
± SD. (cell line 4212, control; cell line 5221, Complex I deficiency
patient 5221 with cardiomyopathy and cataracts.
* p<0.05,
** p<0.01 compared to control cell.)
Figure 13: Aldehyde and acyloin production following increasing doses
of menadione or doxorubicin. Cells were treated with menadione (10, 25 and 50
µM) or doxorubicin (5, 25 and 50 µM) for 2 h. Data presented as mean
± SD. (cell line 4212, control; cell line 5221, Complex I deficiency
patient 5221 with cardiomyopathy and cataracts.
* p<0.05,
** p<0.01 compared to control cell.)
Free radicals and apoptosis in hypoxic-ischemic encephalopathy
Animal studies in hypoxic-ischemic encephalopathy (HIE) have shown that the majority of cell death occurs during the period of reperfusion and reoxygenation. ROS and lipid peroxidation have been thought to be important in the initiation of HIE and the mediation of cell injury and death. Dexamethasone has been shown to significantly reduce the extent of this HIE-induced injury in neonatal rats. The exact mechanisms by which this dose-dependent protection occur are not known. The role of ROS was investigated in this animal model of HIE-induced brain injury to determine the possible role that lipid peroxidation plays in both cell injury and in the protection afforded by dexamethasone pretreatment (6).
Methods
HIE was induced in 7-day old rat pups that were pretreated on day 6 either with 0.1 mg/kg of dexamethasone or phosphate buffered saline. Brain tissue was examined for the evidence of apoptosis 24 hrs following injury. Significant increases in DNA laddering suggestive of apoptosis were observed in treated animals, but were absent in controls and dexamethasone pretreated groups. Since pretreatment with dexamethasone prevents all of the hemispheric tissue loss, this data suggested that apoptosis is the major form of cell death in HIE-induced injury.
Results
Samples taken for analysis 1 h after the onset of reoxygenation of the hypoxic brain tissue were examined for the presence of toxic aldehydes and ROS. Figure 14 shows that animals that were subjected to hypoxic insult had significantly increased levels of total lipid hydroperoxide products (aldehydes) and superoxide compared to normoxic controls. These increases were unaffected by pretreatment with dexamethasone. Other results (data not shown) indicate that the immediate early response gene, c-fos showed a transient increase following hypoxic insult, which was prevented by dexamethasone pretreatment. These data suggest that it is possible to limit hypoxic-ischemic neuronal injury, despite the continued production of reactive oxygen species, by interventions which block the cascade of events that results in apoptosis. Also, the data suggest that the generation of ROS and lipid peroxidation are not the direct mediators of apoptosis in hypoxic-ischemic injury, and that it may be possible to attenuate the damage caused by ROS downstream from their generation (6).
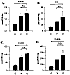 Figure 14: Brain tissue concentrations of aldehyde byproducts of lipid
peroxidation. Panel A: total aldehydes. Panel B: 4-hydroxynonenal. Panel C:
hexanal. Panel D: malondialdehyde. Treatments C: control (no hypoxia). V: hypoxia. D:
hypoxia + dexamethasone.
Figure 14: Brain tissue concentrations of aldehyde byproducts of lipid
peroxidation. Panel A: total aldehydes. Panel B: 4-hydroxynonenal. Panel C:
hexanal. Panel D: malondialdehyde. Treatments C: control (no hypoxia). V: hypoxia. D:
hypoxia + dexamethasone.
Summary and conclusions
The data presented above indicate that hydroxyl radicals, aldehydic products of lipid peroxidation and acyloins (byproducts of aldehyde metabolism) can be measured with good precision and accuracy in plasma and tissues. The methods described above were utilized in the study of a number of animal and human models of disease in which oxidative stress was suspected as a causative agent in the development of injury. Our data suggest that levels of hydroxyl radicals, aldehydes and acyloins are excellent markers for investigating oxidative damage. Concentrations of these markers may change rapidly in-vivo, and their measurement may reveal significant new aspects of the mechanisms by which injury occurs. Our experiments also indicate that free radical production and lipid peroxidation occur early in the cascade of events that leads eventually to injury and cell death. It appears that free radicals and their byproducts might not always be direct mediators of cell death, and that injury may be preventable by interventions downstream from the production of free radicals. The balance between production of ROS and antioxidant reserve also plays an important role in the prevention of free radical-mediated disease and injury.
Acknowledgements
The preparation of this document in HTML format would not have been possible
without the expert technical assistance of Michael Lehotay.
References
- Luo, X.P. and Lehotay, D.C. Determination of hydroxyl radicals using salicylate as a trapping agent by gas chromatography-mass spectrometry. Clin Biochem. 30: 41-46, 1997.
- Luo, X.P., Yazdanpanah, M., Bhooi, N. and Lehotay, D.C., Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry, Analyt Biochem., 228:294-298, 1995.
- Luo, X.P., Evrovsky, Y., Cole, D., Trines, J., Benson, L.N. and Lehotay, D.C. Doxorubicin-induced acute changes in cytotoxic aldehydes, antioxidant status and cardiac function in the rat. Biochim Biophys Acta, 1360:45-52, 1997.
- Luo, X.P., Reichetzer, B., Trines, J., Benson, L.N. and Lehotay, D.C. L-carnitine attenuates doxorubicin induced lipid peroxidation in rats. Free Radical Biol Med. 1998 (in press).
- Luo, X.P., Pitkanen, S, Kassovska-Bratinova, S., Robinson, B.H. and Lehotay, D.C. Excessive Formation of Hydroxyl Radicals and Aldehydic Lipid Peroxidation Products in Cultured, Skin Fibroblasts from Patients with Complex I Deficiency. J Clin Invest., 99:2877-2882,1997.
- Ekert, P., MacLusky, N., Luo X.P., Lehotay D.C., Smith B., Post M. and Tanswell K. Dexamethasone prevents apoptosis in a neonatal model of hypoxic-ischemic encephalopathy (HIE) by a reactive oxygen species-independent mechanism. Brain Res. 747: 9-17, 1997.
| Discussion Board | Previous Page | Your Poster Session |