Poster Contents
| INABIS '98 Home Page | Your Session | Symposia &Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Dopamine (DA) of hypothalamic origin exerts tonic inhibitory controlover the high intrinsic prolactin (PRL) secretory activity of pituitarylactotrophs (1). Dopaminergic (DAergic) perikarya, supplying the pituitarygland with DA, are located in the hypothalamic periventricular (PeVN; A14neurons) and arcuate (ARN ; A12 neurons) nuclei (2). Functionally, theseneurons are grouped according to the course of their projections as periventricularhypothalamic DAergic (PHDA), tuberohypophysial DAergic (THDA), and tuberoinfundibularDAergic (TIDA) neuron populations. PRL, secreted by lactotrophs, acutelystimulates the synthesis of DA in the TIDA and THDA neurons (3) young femalerats, strongly suggesting that PRL regulates its own secretion througha short-loop feedback mechanism. We have recently found that PRL-R is presentin all three neuroendocrine DAergic neuron populations allowing directfeedback by PRL of pituitary origin on all three neuroendocrine DAergicneuron populations (4).
The signal transduction mechanisms associated with the PRL-R are well-characterizedin non-neuronal tissues. Binding of PRL causes dimerization of the PRLreceptor and activation of Jak2 (Janus kinase 2). Jak2 can phosphorylatethe cytoplasmic STAT1, 3 or 5 (signal transducers and activators of transcription)proteins, which leads to subsequent dimerization and nuclear translocationof the STATs. STATs act as transcription factors in the nucleus, leadingto differentiation and/or proliferation. (5). The main STAT protein associatedwith the long form of the PRL-R in non-neural tissues is STAT5 (6). However,it is not known whether the same signal transduction mechanisms are involvedin the activation of PRL-R in the adult brain.
Recently we have characterized the time course and effect of exogenousPRL on the activity of the neuroendocrine DAergic neurons (7). We havefound, that rapid increase in the serum PRL levels were accompanied withsignificant increase in DA turnover in the terminal areas of the TIDA,THDA and PHDA neurons (7).
In order to elucidate the possible mechanisms by which PRL activatesthe hypothalamic DAergic neurons, we investigated the subcellular redistributionof STAT5 protein in these neurons as affected by exogenous PRL administration.
Materialsand Methods
Animals: Two-three month old virgin female Sprague-Dawley rats(Charles River, Raleigh, NC) were ovariectomized under Halothane anesthesia.A group of rats received a subcutaneous injection of 75 microgram/kg BWovine PRL (oPRL-20, AFP#10677C, NIDDK) at 0900 h on the 10th day aftersurgery. The control group received saline only at the same time. The animalswere sacrificed for immunocytochemistry at 1000 h, 1100h or 1300h by anoverdose of Na-pentobarbital and transcardially perfused with ice-coldfixative (4% paraformaldehyde in 0.1 M PBS, pH 7.4). After cryoprotection(20% sucrose-PBS for 24 h) the brains were cut into 30 mm coronal sectionsbetween 600-4200 mm post bregma (pB) with a cryostat (Microm, Zeiss, Germany).
Immunocytochemistry: Sections from both control and oPRL-treated r atswere included in the same assay. After rinsing the sections 3x in PBS andbackground blocking in 10% normal horse serum (in PBS containing 0.4% TritonX 100) for 20 min, the sections were incubated at 4ºC with the antibodiesas described in detail in Table 1.
Table 1: Steps of the double label immunocytochemistry performed inthis study.
| Step | Antibody | Host | Vendor, cat.# | Dilution | Time |
| 1st | anti STAT5 | rabbit | Santa Cruz, sc-835 | 1:800 | 24h |
| 2nd | antirabbit IgG-CY3 | donkey | Jackson,ImmunoRes. 711-165-152 | 1:400 | 18h |
| 3rd | anti TH | mouse | Chemicon, MAB 318 | 1:10000 | 24h |
| 4th | antimouse IgG-CY2 | goat | Jackson,ImmunoRes. 115-225-146 | 1:400 | 18h |
Confocal microscopy: Confocal images were obtained with a Zeiss LSM410 laser-scanning microscope equipped with external Ar/Kr lasers. Sectionsstained in the same assay were scanned with identical parameters. Imageswere scanned from the PeVN (900-1800 mm post bregma and 1800-2100 mm pBdorsal from ARN), the rostral ARN (1800-2100 mm pB), the dorsomedial (DM)and ventrolateral (VL) subpopulations of middle ARN (2100-3600 mm pB) andthe caudal ARN (3600-4200 mm pB) as regions of interest, and from the zonaincerta (A13 neurons) as an internal control area. The images were acquiredwith a Plan-Neofluar 63/1.4 objective lens. The 568 nm laser was attenuatedto 3.3% and the 488 nm laser to 10%.
Radioimmunoassay: Sera from control and oPRL-treated rats (n=5/timepoint/treatment group) were collected after rapid decapitation at everyhalf hour from 0900h to 1100h and every hour from 1100 h to 1400 h on the10th day after surgery. The serum rat and ovine PRL concentrations weredetermined by RIA methods with materials supplied by the NIDDK. The sensitivityof both assays was 1ng/ml. The interassay coefficient of variation was10% and the intraassay coefficient of variation was 5%. The serum PRL concentrationswere statistically evaluated by one factor ANOVA followed by Bonferroni'sm
ultiple comparison test (p<0.05).
Results
Serum levels of oPRL and rPRL: Ovine PRL treatment caused a 10-foldincrease in serum oPRL levels within 1 h, which disappeared after 3 h ofoPRL injection. The rPRL levels decreased significantly 1 h after oPRLinjection, while it was unaffected in the control rats.
Redistribution of STAT5 in the PeVN and ARN: About 60% of the neuronsexhibiting STAT5 immunoreactivity (IR) were also TH-immunopositive in thePeVN and ARN. In the controls STAT5 staining was mainly present in thecytoplasm (Fig.1). One hour after oPRL injection, nuclear translocationof STAT5 can be observed both in the TH-positive (DAergic) and TH-negativeneurons thoughout the ARN (Fig.1).
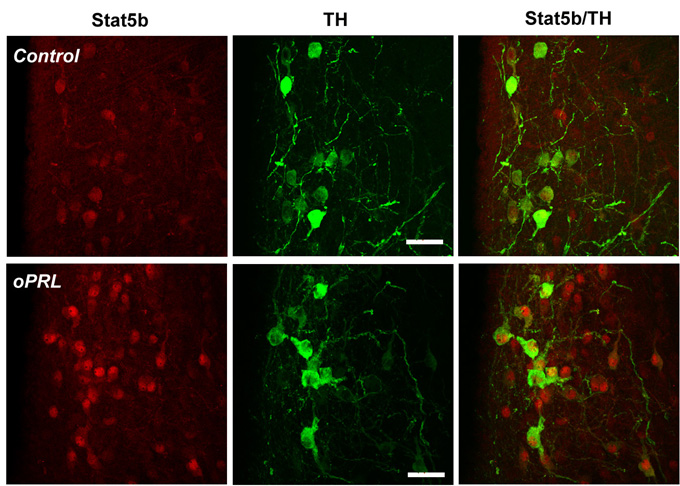
Figure 1: Representative confocal images of the dorsomedial ARN froma control (upper panel) and oPRL-treated (lower panel) rat sacrifized 1h after injection. Bar=25 micrometer.
Interestingly, STAT5 remained mostly cytoplasmic in the PeVN. Two hoursafter oPRL injection nuclear STAT5 staining was still predominant in theTIDA (DM ARN, VL ARN and caudal ARN) and THDA (rostral ARN) neurons. Bythat time a slight increase in nuclear STAT5 staining was found in thePeVN as well, but only a small percentage of PHDA neurons exhibited STAT5staining (Fig.2).
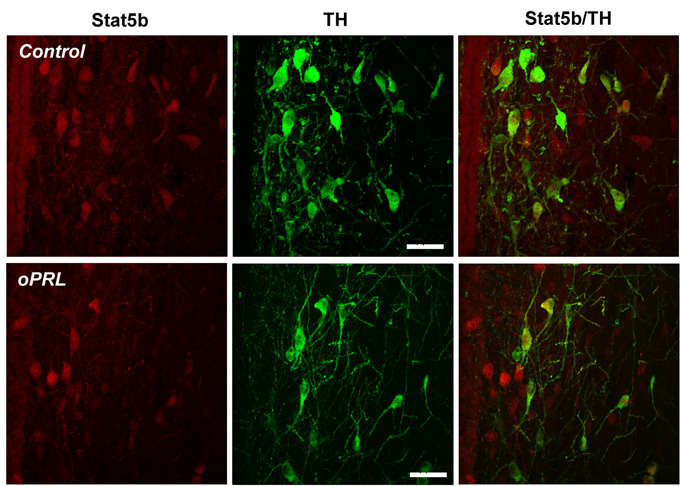
Figure 2: Representative confocal images of the PeVN from a control(upper panel) and oPRL-treated (lower panel) rat sacrifized 2 h after injection.Bar=25 micrometer.
Four hours after the oPRL injection there was no difference in the distributionof STAT5 between the oPRL treated and the c
ontrol groups. Only a very faintcytoplasmic STAT5 staining was detected in the DAergic neurons of the zonaincerta, without significant nuclear translocation in any time point ortreatment group.
Discussionand Conclusion
Ovine PRL treatment resulted in nuclear translocation of STAT5in most of the THDA and TIDA neurons. However, in the PHDA neurons, nucleartranslocation occurred later and in fewer neurons. These data support ourprevious findings that PRL-R are the most abundant in the TIDA and THDApopulations of the ovariectomized rats, and both the PRL-R density andthe number of PRL-R immunoreactive cells are lower in the PHDA neurons(4).Since time course of increase in serum oPRL concentrations and nucleartranslocation of ST AT5 is similar, these data also suggest that a PRL-Rmediated mechanism may be involved in the increased DA turnover of theneuroendocrine DAergic neurons following oPRL administration. Althoughit is unlikely that STAT5 itself as a transcription factor plays a rolein the acute activation of these neurons, it may be a useful marker tofollow the PRL-R mediated events.
1. STAT5 is present in the adult rat hypothalamus and prevalentin the neuroendocrine DAergic neurons.
2. When serum PRL levels are low, STAT5 is localized in the cytoplasmof hypothalamic neurons.
3. Increased serum PRL levels elicit nuclear translocation of STAT5proteins in hypothalamic neurons.
4. The cellular distribution of STAT5 correlates with the density ofPRL-R in different hypothalamic areas.
5. The activity of the neuroendocrine DAergic neurons, especially inthe ARN region are dynamically regulated by the circulating PRL concentrations.The direct effect of PRL on these neurons involves nuclear t
ranslocationof STAT5 protein.
References
1. S.W.J. Lamberts, R.M. MacLeod, Physiol.Rev. 70, 279 (1990).
2. M. Zoli, L.F. Agnati, B. Tinner, H.W.M. Steinbusch, K. Fuxe, J. Chem.Neuroanat. 6, 293 (1993).
3. G.A. Gudelsky, J.C. Porter, Endocrinology 106, 526 (1997).
4. A. Lerant, M.E. Freeman, Brain Res. 802, 141 (1998).
5. V. Goffin, P.A. Kelly, Clinical Endocrinology 45, 247 (1996).
6. V. Goffin, K.T. Shiverick, P.A. Kelly, J.A. Martial, Endocr.Rev.17, 385 (1996).
7. J.E. DeMaria, A. Lerant, M.E. Freeman, Endocrinology submitted, (1998).
| DiscussionBoard | Previous Page | YourPoster Session |