Poster Contents
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Adverse environmental stimuli are known to disrupt early pregnancy by the failure of implantation of fertilized ova into the uterine walls(1-5). When a pregnant female mouse is exposed to a novel male and his secretory products (The Bruce Effect) neuroendocrine events occur in the female, which lead to the disruption of early pregnancy(6). The effect is thought to be pheromonally mediated(7).
Using a double-decker cage system, which houses the exposure males above the pregnant females, we have previously demonstrated that the transmission of the effect is androgen dependent and is not diminished with the surgical removal of the vesicular, coagulating or preputial glands(8,9,10). The effect is greater when the female is housed beneath a greater number of males but diminished when the males are housed beneath the female; suggesting that the pheromone is non-volatile. Sexual satiated males of the same strain (Cf1) and different strain (HS-heterogeneous strain) block pregnancy less effectively than non-satiated males. When urine from strange males is painted directly on the noses of pregnant females, pregnancy is blocked. However, urine collected from a male housed in proximity to a female that he cannot access blocks better than urine from a male housed alone(11). When the feces of the male are prevented from reaching the female the effect is also diminished
In the female, we believe that minute increases in plasma estradiol may be involved in the failure of implantation. Increased plasma estradiol is known to have deleterious effects on implantation(12,13,14); including decreased rate of travel of the fertilized ova down the fallopian tubes as well as inducing lysis of the corpus luteum. If the secretions from the male contain biologically active androgens or estrogens then this may have detrimental effects on implantation in the female who is exposed to these secretions.
Using RIA our lab has found that during the implantation period restraint stressed rats have elevated 17b -estradiol levels(15)and that restraint stressed and Bruce Effect mice given anti-17b -estradiol antibodies implant and continue pregnancy(16,17). We are currently developing urinary and fecal ELISAs which we will use in our Bruce Effect paradigm to determine quantitatively the amounts of androgens and estrogens contained in the male secretions and in the female plasma during implantation.
In order to quantify levels of testosterone, progesterone, pregnandiol, estrone conjugates and 17b -estradiol we have modified a commonly used enzyme linked immunosorbent assay (ELISA) with which we can elucidate the hormonal dynamics of induced pregnancy disruptions. This assay is frequently used at zoos and in field studies in order to assess fertility, sexual maturity and to characterize ovarian hormones and cycles in nondomesticated female animals(18,19,20). It is useful for measuring hormone levels in urine and blood of small, fragile or endangered species, when sampling blood is not practical. It has already been successfully modified for several cat species(21), birds(22), humans(23), and non-human primates(24) but has never been attempted in the common laboratory mouse.
The assay has been validated over the last two years and is currently being used to measure urinary and fecal samples of female mice during early pregnancy and the excretions of the stimulus males. The assay has been validated over the last year and demonstrates several properties of measurement accuracy for fecal and urinary samples. Samples are serially diluted and measured for parallelism against standards of corresponding hormone. An extraction efficiency must also be calculated since not all of the hormone is extracted from the urine and feces. Finally we demonstrate that differences in estradiol and estrone conjugates can be measured in stimulated and non-stimulated males.
Materials and Methods
Sample collection
Urine and fecal samples were collected from 5 pregnant and 5 non-pregnant females over a five day period. The females were housed in groups of 3-4 while 5 males were housed individually all in wire grid bottom cages over a clean surface. The samples were collected between 0900 and 1100 hr and stored at -70° C until analysis.
Sample preparation
Fecal samples were treated with 0.5 ml distilled water, 4.0 ml methanol and 1.0 g of aluminum oxide broken up and turned in test tubes at 16rpm for 1 hour. They were centrifuged at 2500 rpm for 15 min and the supernatant was analyzed for steroid content.
Enzyme immunoassay The enzyme immunoassay for testosterone (test), estradiol (E2) and estrone conjugates (EC) were performed by adding 20ul of each sample to an antibody coated plate. HRP-labeled test, EC and E2 were added immediately after all the samples were added to the plate. After a 2 hr incubation period, the plates were washed and a substrate solution of H2O2 and 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) was added to detect enzyme-labeled steroid. Following colour development, plates were read on a plate reader for optical density. The optical density was converted to a concentration of hormone per gram of feces.
Parallelism and Extraction Efficiency
Serial dilutions were made from the fecal extracts and compared for parallelism with a serially diluted standard curve. The extraction efficiency was determined by spiking the ½ gram of feces with a known concentration of hormone and letting it sit overnight at 4° C. The next day the hormones were extracted and compared to a non-spiked sample. The urine was spiked and sat overnight and measured against non-spiked samples.
Results
For the parallelism, all of the serially diluted samples were parallel to the standard curve. All samples were detectable and reacted with antibody in a similar fashion as the standards. Figure 1 shows pooled fecal samples from pregnant and non-pregnant females (n=10) while Figure 2 shows the same sample analyzed for estrone conjugates. Figures 3-5 show analysis of male urine and feces for testosterone, estradiol and estrone conjugates. Figure 5 illustrates that estrone conjugates in male feces cannot be detected well since the optical density of the most concentrated sample is not in the sensitive range of the curve (the steepest part of the slope).
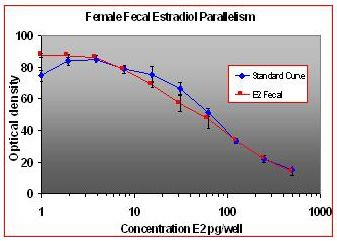 Fig.1: Pooled fecal samples from 5 pregnant and non-pregnant females over a five day period serially diluted to run parallel to estradiol standards.
Fig.1: Pooled fecal samples from 5 pregnant and non-pregnant females over a five day period serially diluted to run parallel to estradiol standards.
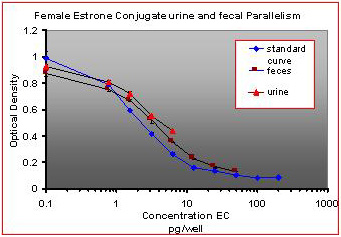 Fig.2 : Pooled fecal samples and urine samples from pregnant and non-pregnant female mice over a five day period serially diluted to run parallel to estrone conjugate standards.
Fig.2 : Pooled fecal samples and urine samples from pregnant and non-pregnant female mice over a five day period serially diluted to run parallel to estrone conjugate standards.
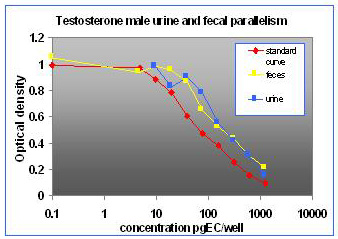 Fig.3: Male mouse fecal and urinary samples tested to run parallel to testosterone standards.
Fig.3: Male mouse fecal and urinary samples tested to run parallel to testosterone standards.
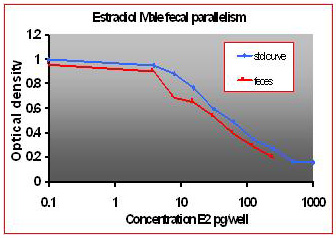 Fig.4: Male mouse fecal and urinary samples tested to run parallel to estradiol standards.
Fig.4: Male mouse fecal and urinary samples tested to run parallel to estradiol standards.
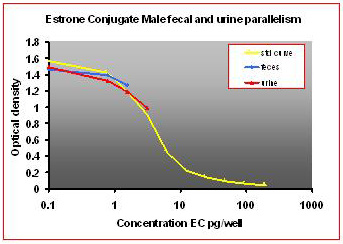 Fig.5: Male mouse fecal and urinary samples tested to run parallel to estrone conjugate standards.
Fig.5: Male mouse fecal and urinary samples tested to run parallel to estrone conjugate standards.
The extraction efficiency was determined by the equation:
% recovered = ((amount measured)/(amount expected))*100
where the amount expected = amount measured in unspiked sample + the calculated amount added.
The efficiency for the extraction of estradiol feces was 77% while for estrone conjugates it was 18%; testosterone has not yet been determined. The recovery for urinary estradiol was 101% while for estrone conjugates it was 100%
Discussion and Conclusion
The fecal and urinary sample curves run parallel to the standard curve and displace the labeled HRP-E2 to give a low optical density. These results demonstrate that since the slopes of the standard and samples are similar, the samples in the urinary and fecal samples will react predictably with the antibody similarly to the standards. Also, since each sample can displace HRP-E2 to under 50% binding, it can be reliably measured within the sensitive range of the standard curve.
Once each assay is validated, samples will be tested from experiments involving the Bruce effect and the disruption of early pregnancy. These experiments will follow our standard paradigm for the Bruce effect as described in earlier experiments. Each assay will involve repeated measures on urine and fecal extracts taken from pregnant female mice in one of three conditions:
1) non-pregnant controls, who will have feces and urine collected on the same days as the other conditions;
2) pregnant, who will have samples collected for the first five days of pregnancy;
3) pregnant Bruce effect, who will be exposed to 2 novel males housed above them and will have samples collected for the first five days of pregnancy. With this assay it will be possible to elucidate the hormonal dynamics of the disruption of implantation and also to reliably measure minute hormonal changes in a non-invasive fashion in the common laboratory mouse. Our lab is also currently adapting this assay for measurement of these same steroids and cortisol in human saliva.
References
1. Liptrap, R. (1993) , Stress and reproduction in domestic animals. Annals New York Academy of Sciences 697: 275-284.
2. Kvetnansky, R.; Sun, C.L.; Lake, C.R.; Thoa, N.; Torda, T.; Koppin, I.J. (1978), Effects of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine and dopamine-b -hydroxylase. Endocrinology 103: 1868-1874.
3. Wettemann, R.P.; Bazer, F.W. (1985), Influence of environmental temperature on prolificacy of pigs. Journal of Reproduction and Fertility, Suppl 33: 199-208
4. Castro-Vazquez, A.; Esquivel, J.; Martin, J.; Rosner, J. (1975), Failure of stressful stimuli to inhibit embryo implantation in the rat. Am J. Obs Gyn 121: 968-970.
5. deCatanzaro, D. (1988), Effect of predator exposure upon early pregnancy in mice. Physiology and Behavior 43: 691-696.
6. Bruce, H.M. (1960), A block to pregnancy in the mouse caused by the proximity of strange males. Journal of Reproduction and Fertility 1: 96-103.
7. Drickamer, L.C. (1989), Pregnancy block in wild stock house mice Mus domesticus: Olfactory preferences of females during gestation. Animal Behavior 37: 690-698.
8. deCatanzaro, D.; Zacharias, R.; Muir, C. (1996), Disruption of early pregnancy by direct and indirect exposure to novel male mice: Comparison of influences of preputialectomized and intact males. Journal of Reproduction and Fertility 106: 269-274.
9. deCatanzaro, D.; Smith, M.; Muir, C. (1995), Strange-male-induced pregnancy disruption in mice: Potentiation by administration of 17b -estradiol to castrated males. Physiology and Behavior 58: 405-407.
10. Zacharias, R.; deCatanzaro, D.; Muir, C. Novel-induced pregnancy disruption in mice: Effects of removal of the vesicular-coagulating complex and the preputial glands of novel males. Physiology and Behavior, Submitted.
11. deCatanzaro, D.; Muir,C.; Sullivan, C.; Boissy, A. Pheromones and novel-male-induced pregnancy disruptions in mice: Exposure to females is necessary for urine alone to induce an Effect. Physiology and Behavior, Submitted.
12. Whitney, R.; Burdick, H. (1936), Tube locking of ova by estrogenic substances. Endocrinology 20: 643-647.
13. Burdick, H.O.; Whitney, R. (1937), Acceleration of the rate of passage of fertilized ova through the fallopian tubes of mice by massive injections of an estrogenic substance. Endocrinology 21: 637-643.
14. deCatanzaro, D.; MacNiven, E.; Ricciuti, F. (1991), Comparison of the adverse effects of adrenal and ovarian steroids on early pregnancy in mice. Psychoneuroendocrinology 16: 525-536.
15. MacNiven, E.; deCatanzaro, D.; Younglai, E.V. (1992), Chronic stress increases oestrogen and other steroids around intrauterine implantation in inseminated rats. Physiology and Behavior 52: 159-162.
16. deCatanzaro, D.; MacNiven, E.; Goodison, T.; Richardson, D. (1994), Estrogen antibodies reduce vulnerability to stress induced failure of intrauterine implantation in inseminated mice. Psychoneuroendocrinology 55: 35-38.
17. deCatanzaro, D.; Muir, C.; O'Brien, J.; Williams, S. (1995), Strange-male-induced pregnancy disruption in mice: Reduction of vulnerability by 17b -estradiol antibodies. Physiology and Behavior 58: 401-404.
18. Peter, A.T.; Kapustin, N.; Critser, J.K. (1996), Analysis of sex steroid metabolites excreted in the feces and urine of nondomesticated animals. Compedium on Continuing Education for the Practicing Veterinarian 18: 781-792.
19. Safar-Hermann, N.; Ismail, M.N.; Choi, H.S.; Mostl, E.; Bamberg, E. (1987), Pregnancy diagnosis in zoo animals by estrogen determination in feces. Zoo Biology 6: 189-193
20. Lasley, B.L.; Kirkpatrik, J.F. (1991), Monitoring ovarian function in captive and free-ranging wildlife by means of urinary and fecal steroids. Journal of Zoo and Wildlife Medicine 22: 23-31
21. Graham, L.H.; Goodrowe, K.L.; Raeside, K.I.; Liptrap, R.M. (1995), Non-invasive monitoring of ovarian function in several felid species by measurement of fecal estradiol-17b and progestins. Zoo Biology 14: 223-237.
22. Lee, J.V.; Whaling, C.S.; Lasley, B.L.; Marler, P. (1995), Validation of an enzyme immunoassay for measurement of excreted estrogen and testosterone metabolites in the White-Crowned sparrow (Zonotrichia leucophrys oriantha). Zoo Biology 14: 97-106.
23. Munro, C.J.; Stabenfeldt, G.H.; Cragun, J.R.; Addiego, L.A.; Overstreet, J.W.; Lasley, B.L. (1991), Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clinical Chemistry 37: 838-844.
24. Carroll, J.B.; Abbott, D.H.; George, L.M.; Hindle, J.E.; Martin, R.D. (1990), Urinary endocrine monitoring of the ovarian cycle and pregnancy in Goeldi's monkey (Callimico goeldii). Journal of Reproduction and Fertility 89: 149-161.
25. Kirkpatrik, J.F.; Lasley, B.L.; Shideler, S.E. (1990), Urinary steroid evaluations to monitor ovarian function in exotic ungulates: VII. Urinary progesterone metabolites in the equidae assessed by immunoassay
26. Czekala, N.M.; Gallusser, J.E.; Meier, J.E.; Lasley, B.L. (1986), The development and application of an enzyme immunoassay for urinary estrone conjugates. Zoo Biology 5: 1-6.
| Discussion Board | Previous Page | Your Poster Session |