![]()
Poster Contents
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Antioxidant N-acetylcysteine Inhibits Development Of Hypoxic Pulmonary Hypertension In Rats
Contact Person: Jan Herget (Jan.Herget@lfmotol.cuni.cz)
Introduction
Chronic hypoxia results in pulmonary hypertension due to an increase in the pulmonary vascular resistance. Remodeling of the peripheral pulmonary arteries is a prominent feature. Vascular smooth muscle cells undergo proliferation and hypertrophy and encroach into the lumen of the alveolar vessels. The turnover of extracellular matrix proteins is increased and shifted towards the deposition of collagen and elastin fibres in the vascular walls (Reid, 1989). The structural reconstruction of the peripheral pulmonary arteries results from the injury to the vascular walls (Herget and Jezek, 1989). Evidence accumulates that the onset of hypoxic pulmonary hypertension is linked to the tissue injury of radical nature. Both nitric oxide (Hampl et al., 1993) and reactive oxygen species (Hoshikawa et al., 1995; Nakanishi et al., 1996) production in the lung is elevated in chronic hypoxia and may contribute to the vascular wall injury. Hoshikawa and co-workers (1995) showed that administration of n-acetylcysteine (NAC), an antioxidant, inhibits the pulmonary vascular oxidative damage and pulmonary hypertension in rats exposed to chronic hypoxia. The oxidant damage was assessed as the level of phosphatidylcholine hydroperoxide measured by chemiluminiscence - HPLC assay. An inhibition of hypoxic pulmonary hypertension was also seen after administration of another antioxidant, dimethylthiourea (Langleben et al., 1989), although the toxicity of the substance makes the interpretation of the mechanism questionable.
In the present study we studied the effects of NAC treatment on the lung hemodynamics in chronic hypoxia.
Materials and Methods
Sixteen rats were exposed to chronic hypoxia (FiO2 = 0.1) in an isobaric hypoxic chamber. Nine of these rats were treated with NAC (20 g/100 ml of drinking water) (group H+NAC). Remaining 7 rats obtained plain water (group H). Sixteen rats were kept in an atmospheric air; 10 obtained NAC in drinking water (group N+NAC), and 6 were normoxic controls (group N). Water consumption was measured every third day and did not differ between the groups.
Immediately after the end of the exposure to hypoxia, the pulmonary artery blood pressure was measured in closed chest rats anesthetized with thiopental (40 mg/kg b.w., i.p.). Then positive pressure ventilation with air was initiated and the chest was opened to measure cardiac output (Transonic Systems) and aortic blood pressure. Then the heart was excised, divided in parts, and weighed.
Results
Rats exposed to chronic hypoxia gained body weight less than normoxic controls. The treatment with NAC did not influence the body weight. Systemic blood pressure and cardiac output were not affected by the sojourn in the hypoxic environment or by the NAC treatment (Table 1).
Table 1
Body weight Aortic blood pressure Cardiac output
[g] [torr] [ml/min]
H 263 ± 21* 111 ± 6 28 ± 5
H+NAC 250 ± 17* 91 ± 5 23 ± 3
N 354 ± 20 107 ± 4 29 ± 4
N+NAC 305 ± 15 103 ± 5 24 ± 2
* = P < 0.001 compared with normoxic controls
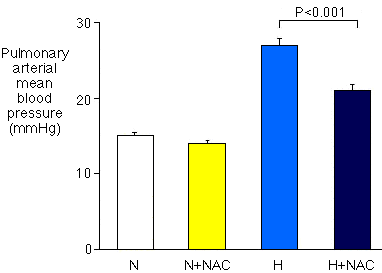
Chronic hypoxia induced pulmonary hypertension, which was inhibited by the NAC treatment. The increase in pulmonary arterial mean blood pressure induced by chronic hypoxia was significantly (P < 0.001) smaller after NAC treatment (Fig. 1). NAC also prevented the increase in the relative weight of the right ventricle (Fig. 2).
 |
|
Figure 1.
|
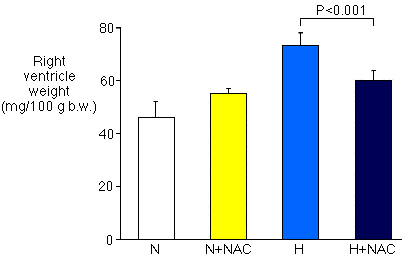 |
| Figure 2. The relative weight of the right heart ventricle is reduced in chronically hypoxic rats treated with N-acetylcysteine (H+NAC) as compared to chronically hypoxic rats with no treatment (H). The right ventricular weight is not significantly different between normoxic rats with (N+NAC) and without (N) N-acetylcysteine treatment. |
Discussion and Conclusion
The main mechanism of the hypoxic pulmonary hypertension is the restriction of the pulmonary vascular bed by the structural remodelling of the peripheral pulmonary vasculature (Reid, 1989). The activation of mesenchymal cells in the vascular walls may be linked to the hypoxic tissue injury (Herget and Jezek, 1989). Production of oxygen radicals is increased during the hypoxic exposure (Nakanishi et al., 1995; Hoshikawa et al., 1995). Vascular changes similar to those seen in chronic hypoxia were noted after the exposure to chronic hyperoxia (Jones et al., 1983).
One possible source of oxygen radicals in chronic hypoxia are alveolar macrophages. Their numbers increase during the hypoxic exposure. In addition, alveolar macrophages are primed by hypoxia to produce more hydrogen peroxide (Tuhoy et al., 1993; Wilhelm et al., 1996).
Radical damage of collagen matrix proteins stimulates the proliferation of vascular smooth muscle "in vitro" (Bacakova et al., 1997). Interstitial collagenases are activated by peroxynitrite (reactive product of the superoxide and nitric oxide interaction) (Rajagopalan et al., 1996). The collagenolytic activity in peripheral pulmonary arteries is increased in chronic hypoxia (Novotna and Herget, 1998). Recently Galis and co-workers (1998) reported that NAC decreases the matrix degrading capacity of macrophages. Collagen breakdown products were shown to stimulate fibroproduction "in vitro" (Gardi et al., 1994) and "in vivo" (Gardi et al., 1990). Inhibition of collagenolytic activity prevents vascular remodelling after injury (Zempo et al., 1996).
We conclude that treatment with NAC inhibits the development of hypoxic pulmonary hypertension, probably due to its interaction with the matrix degrading mechanisms.
References
- Bacakova, L., Wilhelm, J., Herget, J., Novotna, J. and Eckhart, A.: Oxidized collagen stimulates proliferation of vascular smooth muscle cells. Exper. Molec. Pathol. 64: 185-194, 1997.
- Galis, Z. S., Asanuma, K., Godin, D., Meng, X.: N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells. Circulation 97: 2445 - 2453, 1998
- Gardi, C., Pacini, A., de Santi, M. M., Calzoni, P., Viti, A., Corradeschi, F. and al., e.: Development of interstitial lung fibrosis by long-term treatment with collagen breakdpwn products in rabbits. Res. Commun. Chem. Pathol. Pharmacol. 68: 238 - 250, 1990.
- Gardi, D., Calzoni, P., Marcolongo, P., Vavarra, E., Vanni, L. and Lungarella, G.: Collagen breakdown and lung collagen metabolism: an in vitro study on fibroblast cultures. Thorax 49: 312 - 318, 1994.
- Hampl, V., Archer, S. L., Nelson, D. P. and Weir, E. K.: Chronic ERDF inhibition and hypoxia: effects on pulmonary circulation and systemic blood pressure. Journal of Applied.Physiology. 75: 1748-1757, 1993.
- Herget, J. and Jezek, V.: Pulmonary hypertension in chronic lung disease. In Pulmonary Hypertension. Problems and Controversies, ed. by H. Denolin and C. A. Wagenvoort, pp. 142 - 162, Elsevier Publ. Co., 1989.
- Hoshikawa, Y., Ono, S., Tanita, S., Sakuma, T., Noda, M., Tabata, T., Ueda, S., Ashino, Y. and Fujimura, S.: Contribution of oxidative stress to pulmonary hypertension induced by chronic hypoxia. Nippon Kyobu shokkan Gakki Yas 33: 1169-1173, 1995.
- Jones, R., Zapol, W. M. and Reid, L.: Pulmonary arterial wall injury and remodelling by hyperoxia. Chest 83: 40S-42S, 1983.
- Langleben, D., Fox, R. B., Jones, R. C. and Reid, L. M.: Effects of dimethylthiourea on chronic hypoxia-induced pulmonary arterial remodelling and ventricular hypertrophy in rats. Clin. Invest. Med. 12: 235-240, 1989.
- Nakanishi, I., Tajima, F., Nakamura, A., Yagura, S. Y., Ookawara, T., Yamashita, H., Suzuki, K., Taniguchi, N. and Ohno, H.: Effects of hypobaric hypoxia on antioxidant enzymes in rats. J. Physiol. 489: 869-876, 1995.
- Nakanishi, K., Tajima, F., Osada, H., Nakamura, A., Yagura, S., Kawai, T., Suzuki, M. and Torikata, C.: Pulmonary, vascular responses in rats exposed to chronic hypobaric hypoxia at two different altitude levels. Pathol. Res. Pract. 192: 1057-1067, 1996.
- Novotna, J. and Herget, J.: Exposure to chronic hypoxia induces qualitative changes of collagen in the walls of peripheral pulmonary arteries. Life Sci. 62: 1 - 12, 1998.
- Rajagopalan, S., Meng, X. P., Ramasamy, S., Harrison, D. G. and Galis, Z. S.: Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro - Implications for atherosclerotic plaque stability. J. Clin. Invest. 98: 2572-2579, 1996.
- Reid, L.: The control of cellular proliferation in pulmonary circulation. Am. Rev. Resp. Dis. 140: 1490-1493, 1989.
- Tuhoy, M. B., Bain, M. H. and Greening, A. P.: Rat alveolar function is primed by exposure to hypoxia. Eur. Resp. J. 6: 267S, 1993.
- Wilhelm, J., Sojkova, J. and Herget, J.: Production of hydrogen peroxide by alveolar macrophages from rats exposed to subacute and chronic hypoxia. Phys. Res. 45: 185-1991, 1996.
- Zempo, N., Koyama, N., Kenagy, R. D., Lea, H. J. and Clowes, A. W.: Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler. Thromb. Vasc. Biol. 16: 28-33, 1996.
| Discussion Board | Previous Page | Your Poster Session |