Invited Symposium: Neural Bases of Hypnosis
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Hypnotic dissociation of pain dimensions
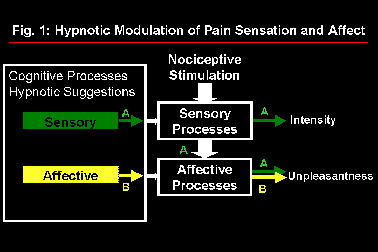
Studies of pain perception have demonstrated the usefulness and validity of the distinction between sensory and affective dimensions both in clinical and experimental contexts (Melzack and Casey, 1968; Price et al., 1987; Price, 1988; Rainville et al., 1992; Fernandez and Turk, 1992). Psychophysical experiments were conducted using an experimental pain model (immersion of the hand in 45.0-47.5°C water for 1 min.) and hypnotic suggestions (Rainville et al., submitted). In one experiment, suggestions designed to increase or decrease pain affect produced the expected modulation of pain unpleasantness with minimal changes in pain intensity (Carrier et al., 1996). In a second experiment, suggestions designed to increase or decrease pain sensation intensity produced parallel changes in both pain intensity and unpleasantness ratings. These results, summarized in Figure 1, are consistent with the successive stage model of pain processing proposed by Wade et al. (1996) to describe clinical pain. The possibility of using hypnotic suggestions to alter specific aspects of the pain experience was thus confirmed. Cerebral correlates of these perceptual changes were investigated using positron emission tomography (PET).
 Click to enlarge
Fig. 1: Model of pain processing in which hypnotic suggestions to modulate pain sensation produce parallel changes in pain intensity and unpleasantness (A) while suggestions directed at pain affect produce specific changes in pain unpleasantness (B).
Click to enlarge
Fig. 1: Model of pain processing in which hypnotic suggestions to modulate pain sensation produce parallel changes in pain intensity and unpleasantness (A) while suggestions directed at pain affect produce specific changes in pain unpleasantness (B).
Cerebral correlates of pain modulation
Painful stimuli activate specific regions of the cerebral cortex. Anatomical and neurophysiological studies indicate that nociceptive signals are relayed to various regions of the cerebral cortex (see Willis and Westlund, 1997). Brain imaging studies in humans have found a reliable pattern of activation using various types of experimental pain stimuli (e.g. Talbot et al., 1991; Jones et al., 1991; Casey et al., 1994, 1996; Coghill et al., 1994; Hsieh et al., 1996; Craig et al., 1996; Aziz et al., 1997). Increases in regional cerebral blood flow (rCBF) are observed in the contralateral primary somatosensory cortex (S1), in the region of the secondary somatosensory cortex (S2)/parietal operculum/posterior insula, in the anterior insula (Insula), and in the anterior cingulate cortex (ACC). These regions were investigated specifically in conditions of hypnotic modulation of pain perception.
Two PET experiments were conducted to evaluate the effect of hypnotic suggestions to alter pain affect (Rainville et al., 1997) or pain sensation (Hofbauer et al., 1998). Pain-related activation in S1, S2, Insula and ACC was confirmed by comparing scans acquired under painfully hot and warm stimulation conditions, both before and after hypnotic induction without suggestions to alter pain perception. Activity in these pain-related sites was compared in conditions of hypnotic modulation of pain affect or pain sensation.
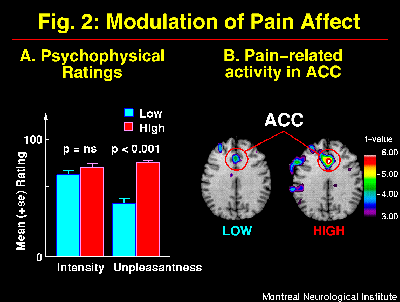
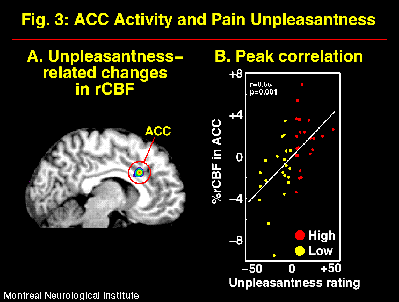
The suggestions for pain affect modulation produced significant and specific changes in pain unpleasantness ratings and in ACC activity (Figure 2; Rainville et al., 1997). Larger pain-related increases in rCBF were observed following suggestions for HIGH than LOW pain unpleasantness. Furthermore, rCBF in ACC was significantly correlated to pain unpleasantness ratings (Figure 3). In both suggestion conditions, pain-related increases in rCBF were also found in S1 and Insula, but not S2. Activity in S1 was slightly higher in the LOW than the HIGH unpleasantness condition (ns), and no differences were observed in the Insula. These results support the hypothesis of a role of the ACC in pain affect.
 Click to enlarge
Fig. 2: Suggestions for decreased (LOW) and increased (HIGH) pain affect produced changes in pain unpleasantness but not pain intensity as indicated by psychophysical ratings (A; also see Figure 1-B). These changes in the experience of pain were accompanied by significant modulation of activity in the ACC as shown in the t-maps of pain-related changes (B). Larger pain-related increases were observed in HIGH than LOW unpleasantness conditions (p<0.05). Pain-related activation was obtained by subtracting rCBF values in the Warm stimulation condition (Hypnosis w/o suggestions) from the Painful stimulation with LOW or HIGH unpleasantness suggestions, respectively. Direct comparison of ACC activation in the two suggestion conditions was performed using volume-of-interest analysis.
Click to enlarge
Fig. 2: Suggestions for decreased (LOW) and increased (HIGH) pain affect produced changes in pain unpleasantness but not pain intensity as indicated by psychophysical ratings (A; also see Figure 1-B). These changes in the experience of pain were accompanied by significant modulation of activity in the ACC as shown in the t-maps of pain-related changes (B). Larger pain-related increases were observed in HIGH than LOW unpleasantness conditions (p<0.05). Pain-related activation was obtained by subtracting rCBF values in the Warm stimulation condition (Hypnosis w/o suggestions) from the Painful stimulation with LOW or HIGH unpleasantness suggestions, respectively. Direct comparison of ACC activation in the two suggestion conditions was performed using volume-of-interest analysis.
 Click to enlarge
Fig. 3: rCBF measured in the ACC was significantly correlated to the psychophysical ratings of pain unpleasantness. A correlation volume (Pearson-r) was derived that revealed a significant peak precisely in the region of pain-related activity in ACC (A). The rCBF values measured at the maximum correlation site in individual scans are plotted against pain unpleasantness ratings in B (residual after Subjects- and intensity-related variance are removed in a linear model; ANCOVA). Each point represent one scan performed in the HIGH (red) or LOW (yellow) unpleasantness condition. The white line shows the linear best fit.
Click to enlarge
Fig. 3: rCBF measured in the ACC was significantly correlated to the psychophysical ratings of pain unpleasantness. A correlation volume (Pearson-r) was derived that revealed a significant peak precisely in the region of pain-related activity in ACC (A). The rCBF values measured at the maximum correlation site in individual scans are plotted against pain unpleasantness ratings in B (residual after Subjects- and intensity-related variance are removed in a linear model; ANCOVA). Each point represent one scan performed in the HIGH (red) or LOW (yellow) unpleasantness condition. The white line shows the linear best fit.
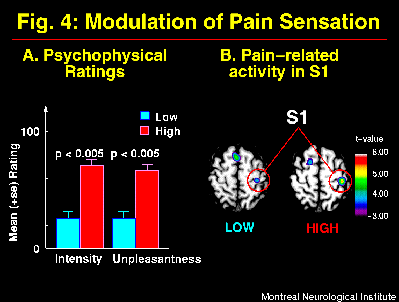
Suggestions directed at the sensory intensity of pain produced parallel modulation of pain intensity and unpleasantness ratings (Figure 4A) consistent with the model shown in Figure 1. These perceptual changes were accompanied by significant changes in brain activity mainly in S1, with larger pain-related increase in rCBF in the HIGH than the LOW pain intensity condition (Figure 4B; Hofbauer et al., 1998). Similar changes were observed in S2 (ns), a smaller effect was found in ACC (ns), and differences in both directions were found in the Insula. These results are consistent with a role of the somatosensory cortex in the sensory dimension of pain perception. Taken together, these results indicate a partial segregation of cerebral regions involved primarily in pain sensory or affective processes, and confirm that hypnotic suggestions to modulate pain alter cerebral activity in regions directly involved in pain perception.
 Click to enlarge
Fig. 4: Suggestions for decreased (LOW) and increased (HIGH) pain sensation produced primary changes in pain intensity and secondary changes in pain unpleasantness as indicated by psychophysical ratings (A; see Figure 1A). These changes in the experience of pain were accompanied by significant modulation of pain-related activity mainly in S1 (B). Brain images show statistical t-maps of significant pain-related changes in conditions of LOW and HIGH pain intensity. Pain-related activation was obtained by subtracting rCBF values in the Warm stimulation condition (Hypnosis w/o suggestions) from the Painful stimulation w/ suggestions.
Click to enlarge
Fig. 4: Suggestions for decreased (LOW) and increased (HIGH) pain sensation produced primary changes in pain intensity and secondary changes in pain unpleasantness as indicated by psychophysical ratings (A; see Figure 1A). These changes in the experience of pain were accompanied by significant modulation of pain-related activity mainly in S1 (B). Brain images show statistical t-maps of significant pain-related changes in conditions of LOW and HIGH pain intensity. Pain-related activation was obtained by subtracting rCBF values in the Warm stimulation condition (Hypnosis w/o suggestions) from the Painful stimulation w/ suggestions.
Previous studies have demonstrated significant changes in physiological activity during hypnotic modulation of pain. Pain-evoked sympathetic activity has been shown to decrease with hypnotically induced analgesia (Lenox, 1970; Hilgard et al., 1974; Rainville et al., submitted). Kiernan et al. (1995) demonstrated that hypnotic analgesia produces a significant reduction in a spinal nociceptive reflex (R-III reflex), as well as in pain intensity and unpleasantness ratings. Modulation of the amplitude of laser-evoked brain potentials has been found in response to hypnotic suggestions of hyperaesthesia and analgesia (Arendt-Nielsen et al., 1990). Pain-evoked potentials have also been recorded using intracranial electrodes implanted in the ACC of a hypnosis responsive patient presenting an obsessive-compulsive disorder (Kropotov et al., 1997). In this study, ratings of pain and distress decreased along with pain-evoked responses in ACC following suggestions for analgesia. Our results further confirm and extend these findings and emphasize the partial segregation of cerebral structures involved in pain sensation and pain affect. Possible cerebral mechanisms involved in the induction of hypnosis and in the response to suggestions have been investigated in a series of post-hoc analyses of the data acquired in the PET study on pain affect modulation (Rainville et al., in press).
Hypnotic relaxation and suggestions
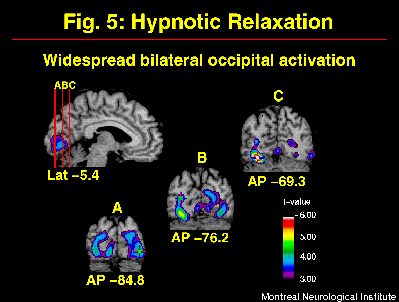
Neuropsychological studies have suggested that hypnosis relies largely on right hemisphere function (e.g. Carter et al., 1982; Gabel, 1988). The comparison of the distribution of rCBF before and after hypnotic induction (w/o suggestions for pain modulation) allowed for the evaluation of this hypothesis (warm and painful stimulation conditions pooled). Changes in rCBF included bilateral increases in occipital and inferior frontal cortices (Figure 5). Significant decreases were found in bilateral medial and in right lateral aspects of the posterior parietal lobes (see Rainville et al. in press for other significant changes). This bilateral pattern of activity indicates that hypnosis largely involves the left hemisphere, in agreement with more recent models of hypnosis (e.g. Jasiukaitis et al., 1997). Although a definite interpretation of the pattern of increases and decreases in rCBF is beyond the scope of these experiments, interesting similarities with other studies can be outlined.
 Click to enlarge
Fig. 5: Hypnotic induction produced strong and widespread rCBF-increases in occipital cortices. Statistical t-map of significant Hypnotic induction-related changes was obtained by subtracting scans in the Awake control condition from scans acquired after the induction of hypnosis (w/o suggestions to modulate pain).
Click to enlarge
Fig. 5: Hypnotic induction produced strong and widespread rCBF-increases in occipital cortices. Statistical t-map of significant Hypnotic induction-related changes was obtained by subtracting scans in the Awake control condition from scans acquired after the induction of hypnosis (w/o suggestions to modulate pain).
Instructions for relaxation used in the present study are part of most hypnotic induction procedures. Other states of deep relaxation have been shown to produce changes in rCBF, some of which are consistent with our results. For example, bilateral increases in occipital activity have been observed during deep stages of sleep (Hofle et al., 1997), meditation (Kjaer et al., 1997), and during a gradual decrease in arousal in a prolonged auditory vigilance task (Paus et al., 1997). One possible explanation for such increase in activity is a decrease in inhibitory mechanisms. Cross-modality suppression has been suggested to explain the decreased activity in visual cortices during the processing of stimuli in another modality, as a mechanism for selective attention (Kawashima et al., 1995). Such suppression may be stronger in the awake control condition during the processing of the thermal stimulus. A release from such suppression during hypnosis could lead to a facilitation of visual imagery processes which have been shown to depend on occipital activity (D'Esposito et al., 1997; Kosslyn et al., 1995). This mechanism would be consistent with spontaneous imagery reported in some subjects of our studies, and might underlie the high levels of imagery vividness reported in highly hypnotizable subjects (Crawford, 1982).
Hypnotic induction also produced reliable decreases in rCBF in parietal cortices and increases in the right caudal ACC (+4.6) and frontal cortices (Rainville et al., in press). This constellation of cortical structures has been suggested to be part of an attentional system (Posner and Petersen, 1990). However, the experimental conditions in which subjects were scanned differ markedly from other studies in which cognitive demanding tasks are administered to evaluate attentional abilities in relation to hypnosis (e.g. Crawford, Brown, and Moon, 1993). Nevertheless, it is possible that the observed pattern of activity reflects decreased attention and orientation to irrelevant extra-personal stimuli following hypnotic induction. Moreover, the right lateralization of the ACC increase and the lateral parietal decrease could relate to the asymmetric effect of hypnosis on performance observed in various neuropsychological tasks (e.g. Carter et al., 1982).
Alternatively, the caudal part of ACC has also been associated with hand/arm motor control (see Paus et al., 1998). Increases in rCBF have been observed in the pre-motor cortex and supplementary motor area in response to instructions for muscular relaxation (Tomas,1998). We cannot exclude the possibility that the activation found in ACC during hypnosis could reflect similar motor control processes.
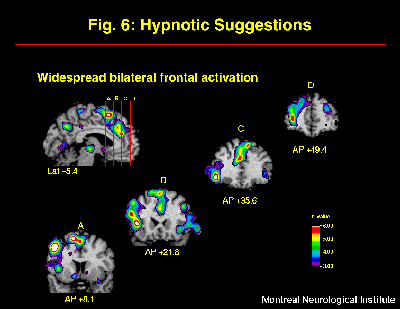
The cerebral activity observed following hypnotic induction alone was markedly modified by the additional inclusion of suggestions for pain modulation (Rainville et al, in press). The general effect of suggestions was evaluated by subtracting rCBF values acquired in the Hypnosis alone with painful stimulus presentation, from those observed in the Hypnosis-with-Suggestion conditions (increased and decreased pain unpleasantness conditions pooled). The main effect observed was a widespread bilateral increase in frontal rCBF (left > right), predominant within medial superior and left dorso-lateral aspects (Figure 6). This strong increase in frontal activity following hypnotic suggestions for pain modulation replicated and extended previous findings by Crawford et al. (1993). Increases in rCBF were also found in medial and lateral posterior parietal cortices. Both the increased and decreased pain unpleasantness suggestion conditions contributed to these effects.
 Click to enlarge
Fig. 6: Suggestions for pain modulation produced strong and widespread increase in rCBF mainly in bilateral frontal cortices. Statistical t-map of significant Suggestions-related changes was obtained by subtracting scans acquired following the induction of hypnosis (w/ painful stimulation) from scans acquired in both suggestions conditions (painful stimulation with HIGH or LOW pain unpleasantness conditions pooled).
Click to enlarge
Fig. 6: Suggestions for pain modulation produced strong and widespread increase in rCBF mainly in bilateral frontal cortices. Statistical t-map of significant Suggestions-related changes was obtained by subtracting scans acquired following the induction of hypnosis (w/ painful stimulation) from scans acquired in both suggestions conditions (painful stimulation with HIGH or LOW pain unpleasantness conditions pooled).
The frontal lobe has a likely contribution to many functions involved in the integration and actualization of the suggestions for perceptual modulation. Activation of the frontal lobe may reflect the active verbal processing of the suggestions, consistent with its role in lexical-semantic analysis (e.g. Petersen et al., 1989; Klein et al., 1995) and working memory (Paulesu et al., 1993; D'Esposito et al., 1995). Reality testing and the monitoring of present external event have also been associated with prefrontal function (Knight and Grabowecky, 1995). The left frontal lobe may be involved in internally generated, or top-down, reinterpretation of the painful stimulus and/or the general experimental context, consistent with previous explanations (Gazzaniga, 1989; Phelps and Gazzaniga, 1992; Jasiukaitis et al., 1997). The hypnotic suggestions for pain modulation given in the present experiment were precisely aimed at altering the perceived external reality by reinterpretation of the pain sensations. Although different types of hypnotic suggestions would probably lead to distinct patterns of activation, frontal activation might be common to the actualization of various types of suggestions involving alteration of the meanings of perceptual experiences. The neurophysiological mechanism for such modulation could include descending inhibition (e.g. Kiernan et al., 1995), frontal modulatory control of thalamo-cortical activity (e.g. Crawford et al., 1998), and/or cortico-cortical interactions. The specific neurophysiological systems involved likely depend on the nature of the alteration of the conscious experience.
Conclusions
Hypnotic suggestions have provided a powerful tool to study the anatomo-functional correlates of pain sensory and affective processes. Differential modulation of pain intensity and unpleasantness produced changes in specific cortical areas. These effects are taken as evidence of a partial segregation of cerebral regions involved in pain sensory and affective processes. Moreover, results confirm the action of hypnotic suggestions on pain-related areas. Changes associated with the induction of hypnosis are consistent with the production of a state of relaxation that may facilitate internally generated imaginative processes through the reduction of inhibitory mechanisms such as cross-modality suppression. On the other hand, suggestions to alter perception produced strong changes in frontal cortices that may reflect the contribution of verbal processes and the active biasing of the perceptual experience. The speculative nature of these interpretations needs to be acknowledged. However, future brain imaging studies testing specific hypotheses may contribute significantly to the understanding of the neurophysiological mechanisms underlying the hypnotic modulation of conscious experience.
References
- Arendt-Nielsen, L, Zachariae, R, and Bjerring, P (1990) Quantitative evaluation of hypnotically suggested hyperaesthesia and analgesia by painful laser stimulation. Pain, 42: 243-251.
- Aziz, Q, Andersson, JL, Valind, S, Sundin, A, Hamdy, S, Jones, AK, Foster, ER, Langstrom, B, and Thompson, DG (1997) Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology, 113: 50-59.
- Carrier, B, Rainville, P, Duncan, GH, and Bushnell, MC (1996) Hypnotic (affective) analgesia during tonic heat pain. International Association for the Study of Pain, Abst. #134.
- Carter, BD, Elkins, GR, and Kraft, SP (1982) Hemispheric asymmetry as a model for hypnotic phenomena : A review and analysis. The American Journal of Clinical Hypnosis, 24: 204-210.
- Casey, KL, Minoshima, S, Berger, KL, Koeppe, RA, Morrow, TJ, and Frey, KA (1994) Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. Journal of Neurophysiology, 71: 802-807.
- Casey, KL, Minoshima, S, Morrow, TJ, and Koeppe, RA (1996) Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. Journal of Neurophysiology, 76: 571-581.
- Coghill, RC, Talbot, JD, Evans, AC, Meyer, E, Gjedde, A, Bushnell, MC, and Duncan, GH (1994) Distributed processing of pain and vibration by the human brain. Journal of Neuroscience 14: 4095-4108.
- Craig, AD, Reiman, EM, Evans, A, and Bushnell, MC (1996) Functional imaging of an illusion of pain. Nature, 384: 258-260.
- Crawford, HJ (1982) Hypnotizability, daydreaming styles, imagery vividness, and absorption: a multidimensional study. Journal of Personality and Social Psychology, 42: 915-926.
- Crawford, HJ, Brown, AM, and Moon, CE (1993) Sustained attentional and disattentional abilities: differences between low and highly hypnotizable persons. Journal of Abnormal Psychology, 102: 534-543.
- Crawford, HJ, Gur, RC, Skolnick, B, Gur, RE, and Benson, DM (1993) Effects of hypnosis on regional cerebral blood flow during ischemic pain with and without suggested hypnotic analgesia. International Journal of Psychophysiology 15: 181-195.
- Cawford, HJ, Knebel, T, Kaplan, L, Vendemia, MC, Xie, M, Jamison, S, and Pribram, KH (1998) Hypnotic analgesia: 1. Somatosensory event-related potential changes to noxious stimuli and 2. Transfer learning to reduce chronic low back pain. The International Journal of Clinical and Experimental Hypnosis, 46: 92-132.
- D'Esposito, M, Detre, JA, Aguire, GK, Stallcup, M, Alsop, DC, Tippet, LJ, and Farah, MJ (1997) A functional MRI study of mental image generation. Neuropsychologia. 35: 725-30.
- D'Esposito M, Detre, JA, Alsop, DC, Shin, RK, Atlas, S, and Grossman, M (1995) The neural basis of the central executive system of working memory. Nature, 378: 279-281.
- Fernandez, E, and Turk, DC (1992) Sensory and affective components of pain: separation and synthesis. Psychological Bulletin, 112: 205-217.
- Gabel, S (1988) The right hemisphere in imagery, hypnosis, rapid eye movement sleep and dreaming. Empirical studies and tentative conclusions. Journal of Nervous & Mental Disease, 176: 323-331.
- Gazzaniga, MS (1989) Organization of the human brain. Science, 245: 947-952.
- Hilgard, ER, Morgan, AH, Lange, AF, Lenox, JR, MacDonald, H, Marshall, GD, and Sachs, LB (1974) Heart rate changes in pain and hypnosis. Psychophysiology, 11: 692-702.
- Hofbauer, RK, Rainville, P, Duncan, GH, and Bushnell, MC (1998) Modulation of pain sensation alters activity in human cerebral cortex. Society for Neuroscience Abstract, 24: Abstr. #447.5.
- Hofle, N, Paus, T, Reutens, D, Fiset, P, Gotman, J, Evans AC, and Jones BE (1997) Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. Journal of Neuroscience, 17: 4800-4808.
- Hsieh, JC, Stahle-Backdahl, M, Hagermark, O, Stone-Elander, S, Rosenquist, G, and Ingvar, M (1996) Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain, 64: 303-314.
- Jasiukaitis, P, Nouriani, B, Hugdahl, K, and Spiegel, D (1997) Relateralizing hypnosis: Or, have we been barking up the wrong hemisphere? The International Journal of Clinical and Experimental Hypnosis, 45: 158-177.
- Jones AK, Brown, WD, Friston, KJ, Qi, LY, and Frackowiak, RS (1991) Cortical and subcortical localization of response to pain in man using positron emission tomography. Proceedings of the Royal Society, London - Series B: Biological Science, 244: 39-44.
- Kawashima, K, O'Sullivan, BT, and Roland, PE (1995) Positron-emission tomography studies of cross-modality inhibition in selective attentional tasks: closing the "mind's eye". Proceedings of the National Academy of Sciences of the United States of America, 92: 5969-5972.
- Kiernan, BD, Dane, JR, Phillips, LH, and Price, DD (1995) Hypnotic analgesia reduces R-III nociceptive reflex: further evidence concerning the multifactorial nature of hypnotic analgesia. Pain, 60: 39-47.
- Kjaer, TW, Lou, HC, Nowak, M, Wildschi»dtz, G, and Friberg, L (1997) Consciousness quality and quantity. Society for Neuroscience Abstracts, 23: Abstr. #192.12.
- Klein, D, Milner, B, Zatorre, RJ, Meyer, E, and Evans AC (1995) The neural substrates underlying word generation: a bilingual functional-imaging study. Proceedings of the National Academy of Sciences of the United States of America, 92: 2899-2903.
- Knight, RT, and Grabowecky, M (1995) Escape from linear time: prefrontal cortex and conscious experience. In The cognitive neuroscience. MS Gazzaniga (Ed.), MIT press, Cambridge, Mass., pp. 1357-71.
- Kosslyn, SM, Thompson, WL, Kim, IJ, and Alpert, NM (1995) Topographical representations of mental images in primary visual cortex. Nature, 378: 496-498.
- Kropotov, JD, Crawford, HJ, and Polyakov, YI (1997) Somatosensory event-related potential changes to painful stimuli during hypnotic analgesia: anterior cingulate cortex and anterior temporal intracranial recordings. International Journal of Psychophysiology, 27: 1-8.
- Lenox, JR (1970) Effect of hypnotic analgesia on verbal report and cardiovascular responses to ischemic pain. Journal of Abnormal Psychology, 75: 199-206.
- Melzack, R, and Casey, KL (1968) Sensory, motivational, and central determinants of pain: A new conceptual model. In D Kenshalo (Ed.), The Skin Senses. Thomas, Springfield IL, pp. 423-443.
- Paulesu, E, Frith, CD, and Frackowiack, SJ (1993) The neural correlate of the verbal component of working memory. Nature, 362: 342-345.
- Paus, T, Koski, L, Caramanos, Z, and Westbury, C (1998) Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport, 9: R37-R47.
- Paus, T, Zatorre, RJ, Hofle, N, Caramanos, Z, Gotman, J, Petrides, M, and Evans, A (1997) Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. Journal of Cognitive Neuroscience, 9: 392-408.
- Petersen, SE, Fox, PT, Posner, MI, Mintun, M, and Raichle, ME (1989) Positron emission tomographic studies of the processing of single words. Journal of Cognitive Neuroscience, 1: 153-170.
- Phelps, EA, and Gazzaniga, MS (1992) Hemispheric differences in mnemonic processing: the effect of left hemisphere interpretation. Neuropsychologia, 30: 293-297.
- Posner, MI, and Petersen, SE (1990) The attention system of the human brain. Annual Review in Neuroscience, 13: 25-42.
- Price, DD (1988) Psychological and neural mechanisms of pain. Raven Press, New York.
- Price, DD, Harkins, SW, and Baker, C (1987) Sensory-affective relationships among different types of clinical and experimental pain. Pain, 28: 297-307.
- Rainville, P, Duncan, GH, Price, DD, Carrier, B, and Bushnell, MC (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science, 277: 968-970.
- Rainville, P, Feine, JS, Duncan, GH, and Bushnell, MC (1992) A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosensory and Motor Research, 9: 265-277.
- Rainville, P, Hofbauer, RK, Paus, T, Duncan, GH, Bushnell, MC, and Price, DD (in press). Cerebral mechanisms of hypnotic induction and suggestion. Journal of Cognitive Neuroscience.
- Rainville, P, Carrier, B, Hofbauer, RK, Bushnell, MC, and Duncan, GH (submitted). Dissociation of pain sensory and affective processes using hypnotic modulation.
- Talbot, JD, Marrett, S, Evans, AC, Meyer, E, Bushnell, MC, and Duncan, GH (1991) Multiple representations of pain in human cerebral cortex. Science, 251: 1355-1358.
- Tomas, K, Hanakawa, T, Honda, M, Fukuyama, H, Okada, T, Nishizawa, S, Konishi, J, and Shibasaki, H (1998) Brain areas associated with voluntary muscle relaxation, single trial event-related fMRI study. Neuroimage, 7: S950.
- Wade, JB, Dougherty, LM, Archer, CR, and Price, DD (1996) Assessing the stages of pain processing: a multivariate analytical approach. Pain, 68: 157-167.
- Willis, WD and Westlund, KN (1997) Neuroanatomy of the pain system and of the pathways that modulate pain. Journal of Clinical Neurophysiology, 14: 2-31.
| Discussion Board | Previous Page | Your Symposium |