Invited Symposium: Iron Transport
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Iron is required by cells for the synthesis of many proteins and enzymes needed for essential cellular processes such as oxygen transport, energy production and cell proliferation. It is absorbed from the diet mainly by the duodenum and transported in the plasma bound to either transferrin or low molecular weight complexes such as citrate. Most types of cells acquire iron from both sources and it must be transported across the cell membrane by iron carriers. Until recently little has been known about these carriers. However, using expression cloning of mRNA from rat duodenum in Xenopus oocytes, Gunshin et al (1) identified a gene that encodes a transmembrane protein that is capable of transporting iron across cell membranes. It is now called DCT1 or Nramp2. It is a 561 amino acid protein consisting of 12 putative transmembrane spanning domains which can transport a number of other divalent metal-ion including cobalt, zinc, manganese, cadmium, copper, nickel and lead. Metal transport is coupled to H+ transport and is dependent on cell membrane potential. Nramp2 mRNA was shown to be widely expressed in many tissues with highest levels in the duodenum, brain, bone marrow and kidney and lower levels in other tissues including the colon, liver and testis.
Studies by Fleming et al (2, 3) using microcytic anaemic mice (mk) and Belgrade rats (b) have provided additional evidence that Nramp2 plays a role in iron transport. Both of these animals have inherited anaemia due impaired iron absorption and defective iron transport in developing erythoid cells caused by a defective iron carrier (4-7). Using positional cloning Fleming et al, found that both the mk mouse (2) and the Belgade rat (3) have a missense mutation (G185A) in Nramp2. Functional studies using human embryonic kidney cells transfected with either the wild type or the mutant form of Nramp2 showed that the mutation caused a reduction in Nramp2 protein levels as well as the complete disruption to cellular iron transport (8).
In order to obtain a greater understanding of the function of Nramp2 in iron transport it is important to determine the tissue and cellular distribution of the protein. Therefore, we have produced an antibody to rat Nramp2 to examine its expression in tissues that play an important role in iron metabolism such as the duodenum, bone marrow, liver, colon and kidney using immunohistochemistry.
Materials and Methods
Animals: Male Wistar rats were fed a control diet containing 70 mg iron/kg diet in the form of ferrous ammonium sulphate for 8 to 10 weeks from the time of weaning. The animals were killed by injection of Nembutal (Boehringer Ingleheim, Sydney, Australia) at a dose of 100mg/kg.
Production of Nramp2 antibodies: A 14 amino acid peptide was synthesized according to the predicted amino acid sequence (codon 230-243) obtained from the rat Nramp2 cDNA (1). This peptide is located in the extracellular loop 3 of the protein and contains only 6 amino acids in common with Nramp1. The peptide (350 µg) was conjugated to bovine serum albumin using activated malemide (Pierce, Sydney, Aust.). Antibodies to the conjugate were raised in rabbits using standard procedures. Production of antibodies against the peptide was confirmed by ELISA. Antibodies to bovine serum albumin were removed from the antiserum by affinity chromatography using CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech, Melbourne, Aust). Removal of antibodies against bovine serum albumin was confirmed by ELISA.
Morphological studies: The duodenum, colon, pancreas, liver and kidney were removed from the animals and immediately placed into buffered formol saline (pH 7.4). The tissues were paraffin embedded and 5 µm sections were cut, floated onto slides coated with 0.5% gelatine and allowed to dry. The sections were then dewaxed, rehydrated in phosphate buffered saline (PBS) and used in the immunohistochemical procedures described below.
Immunohistochemistry: The rehydrated tissue sections were reacted with 2.5% aqueous periodic acid for 5 min and 0.02% sodium borohydride for 2 min to remove endogenous peroxidase activity. The sections were incubated with PBS containing 0.2% saponin, and then with the Nramp2 antibody diluted 1:500 with PBS-saponin overnight at 4°C. They were washed three times with PBS-saponin followed by incubation with a sheep anti-rabbit IgG peroxidase-coupled secondary antibody diluted 1:100 with PBS-saponin (Silenus, Amrad Biotech, Melbourne, Aust.) for 2 hr at room temperature, followed by a further three washes with PBS-saponin. The protein was detected with 0.05% 3’, 3’-diaminobenzidine tetrahydrochloride and 0.01% peroxidase. The tissues were dehydrated and mounted in DePeX (BDH, Melbourne, Aust.). Sections used for control staining were processed by the same method except that pre-immune rabbit antiserum was substituted for Nramp2 antiserum. The specificity of the Nramp2 antibody was determined by incubating sections with the Nramp2 antibody (1:500) in the presence of 50µg Nramp2 peptide at the same time as other sections were incubated with the antibody in the absence of the peptide before processing as described above.
Results
Localisation of Nramp2 protein: Nramp2 protein was expressed in many tissues including the duodenum, colon, bone marrow, kidney, pancreas and liver. The intensity of staining was greatest in the duodenum, kidney and bone marrow with less intense staining in the colon, pancreas and liver.
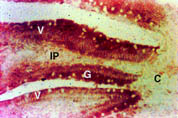
Duodenum: Nramp2 protein was detected in the villus enterocytes of the duodenum. As can be seen in Figure 1, there was strong staining extending from the crypt-villus junction to the villus tip (V). Nramp2 protein was not detected in the crypt epithelial cells (C). The staining was specific for enterocytes as no staining was observed in the goblet cells (G) or the lamina propria (LP) including the duodenal macrophages.
Nramp2 was distributed evenly throughout the cytoplasm of the villus enterocytes and there was a low level of margination of the protein to the apical cell membrane. The nucleus stained negative. The cytoplasm and apical membrane did not stain when Nramp2 antibody was used in the presence of the Nramp2 peptide or using pre-immune antiserum.
 Click to enlarge
Fig. 1: Localisation of Nramp2 in the duodenum.
Click to enlarge
Fig. 1: Localisation of Nramp2 in the duodenum.
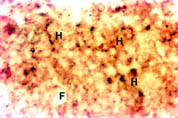
Colon: Nramp2 protein was detected in the villus colonocytes. The protein was found in the cytoplasm of the cells but there was no margination of the protein to the membrane lining the luminal surface of the colon. There was no detectable expression of Nramp2 protein in the crypt epithelium of the colon. Bone Marrow: Haematopoietic tissue was taken from the bone marrow of the femur by aspiration. Haemopoietic cells (H) stained positively for Nramp2 while, fat cells (F) were negative (Figure 2).
 Click to enlarge
Fig. 2: Expression of Nramp2 in the bone marrow.
Click to enlarge
Fig. 2: Expression of Nramp2 in the bone marrow.
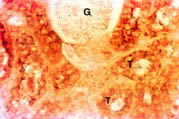
Kidney: There was intense staining for Nramp2 in the cortex (Figure 3) and medulla (data not shown) of the kidney. Nramp2 protein was detected in the proximal, distal and collecting tubules (T) of the nephron while, the glomerulus (G) was negative.
 Click to enlarge
Fig. 3: Distribution of Nramp2 in the cortex of the kidney.
Click to enlarge
Fig. 3: Distribution of Nramp2 in the cortex of the kidney.
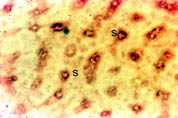
Liver: As shown in Figure 4, Nramp2 protein was detected in the cells lining the liver sinusoids (S). This pattern of staining was consistent with the presence of the protein on the microvillus membrane of the hepatocytes although its presence in endothelial cells cannot be excluded. The Kupffer cells, the macrophages of the liver, did not stain. To determine if the Nramp2 was expressed by the hepatocytes lining the sinusoids, we performed immunohistochemical staining for Nramp2 on isolated hepatocytes. These cells expressed Nramp2 on the cell surface membrane but no intracellular expression of protein was detected (data not shown).
 Click to enlarge
Fig. 4: Localisation of Nramp2 in the liver.
Click to enlarge
Fig. 4: Localisation of Nramp2 in the liver.
Pancreas: The pancreas stained positively for Nramp2 (Figure 5). The protein co-localised with insulin to the islets of Langerhans (I). Nramp2 was not detected in the acinar or ductular cells in the exocrine tissue of the pancreas.
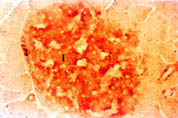 Click to enlarge
Fig. 5: Expression of Nramp2 in the pancreas.
Click to enlarge
Fig. 5: Expression of Nramp2 in the pancreas.
Discussion and Conclusion
The Nramp2 protein was expressed in many tissues. The intensity of staining was greatest in the duodenum and the bone marrow, tissues that play a major role in iron metabolism. The cellular distribution and level of expression of the protein was similar to that obtained for the Nramp2 mRNA determined by in situ hybridisation and Northern analysis (1). Specificity of the antibody for Nramp2 was confirmed by the inhibition of staining when the antibody was used in the presence of excess of the Nramp2 peptide. The antibody was also specific for Nramp2 as it did not cross react with the closely related protein Nramp1. Nramp1 is only expressed in macrophages (9) and the Nramp2 antibody did not stain the macrophages in either the lamina propria in the duodenum or the Kupffer cells in the liver.
The villus enterocytes in the duodenum are absorptive cells capable of transporting a number of transient metals including iron from the intestinal lumen into the cell and transferring them to the plasma. It has been proposed that there are two carriers in these intestinal cells, one on the apical surface which transports metals into the cell and the other on basolateral membrane that transports metals from the cells to the plasma. Nramp2 protein was expressed in high levels in the villus region consistent with a role in metal absorption. Most of the protein was located inside the cell with a low level of the protein expression on the apical cell surface. There was no evidence of the protein on the basolateral surface. The cellular localisation of the protein suggests that it is probably the apical cell surface iron carrier. There was no evidence of the protein in the crypt cells. This was surprising, as these cells express transferrin receptors and take up iron from the plasma (10). It is possible that the expression of Nramp2 is very low in these cells so that it cannot be detected by immunohistochemistry.
In the colon the cellular distribution of Nramp2 was similar to that observed in the duodenum. However, the intensity of staining was less in the colon. Unlike the duodenum, Nramp2 was expressed only inside the cells and there was no expression on the luminal cell surface which may be related to the fact that the colonocytes do not absorb metal ions.
Approximately 70% of the plasma iron turnover is taken up by the haemopoietic cells of the bone marrow. Most of this iron is incorporated into the developing erythrocytes for the synthesis of haemoglobin by transferrin receptor-mediated endocytosis. Consistent with this, Nramp2 protein was expressed highly by the haemopoietic cells in the bone marrow. This supports the conclusion of Fleming et al (2, 3), based on studies with the mk mouse and Belgrade rat, that Nramp2 is likely to be the endosomal iron transporter.
There was a high degree of expression of Nramp2 in the epithelial cells lining the renal tubules. These results suggest that Nramp2 has a function in the reabsorption of metals by the kidney. Most of the iron in the plasma is transported by the protein transferrin which largely prevents filtration at the glomerulus and therefore it is unlikely that reabsorption plays a significant part in normal iron metabolism. However, it is possible that other metals such as manganese and zinc which are less strongly bound by plasma proteins will be filtered at the glomerulus and reabsorbed across the luminal cell membrane of the nephron by Nramp2.
Hepatocytes of the liver are the main site for storage of excess iron and they can take up iron in many forms including transferrin-bound iron and nontransferrin-bound iron (11). Nramp2 was detected on the plasma cell surface of hepatocytes lining the sinusoids, suggesting Nramp2 was involved in the transmembrane transport of iron from the plasma to the hepatocyte.
Nramp2 was expressed in low levels by the Islets of Langerhans in the pancreas. Little is known about metal transport in the pancreas. However, the islets are a target for iron overload in genetic haemochromatosis and damage to these cells by excess iron can cause diabetes mellitus.
In summary we have shown, using a specific antibody produced against a Nramp2 peptide, that the level of expression of Nramp2 protein and its tissue and cellular distribution are consistent with a role in the absorption by the duodenum, reabsorption in the kidney and the transmembrane transport of iron by haemopoietic, liver and pancreatic cells.
ACKNOWLEDGMENTS
This work was supported by grants from the National Health and Medical Research Council of Australia and The Raine Medical Research Foundation.
References
- Gunshin H., Mackenzie B., Berger U.V., Gunshin Y., Romeo M.F., Boron W.F., Nussberger S., Gollan J.L. and Hediger M.A. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482-488.
- Fleming M.D., Trenor C.C., Su M.A., Foernzler D., Beier D.R., Dietrich W.F., and Andrews N.C. (1997) Microcytic anaemia mice have a mutation in Nramp2 a candidate iron transporter gene. Nature Genetics 16, 383-386.
- Fleming M.D., Romano M.A., Su M.A., Garrick L.M, Garrick M.D. and Andrews N.C. (1998) Nramp2 is mutated in the anemic belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl. Acad. Sci. USA. 5, 1148-1153.
- Edwards J.A. and Hoke J.E. (1972) Defects of intestinal mucosal iron uptake in mice with hereditary microcytic anemia. Proc. Soc. Exp. Biol. Med. 141, 81-84.
- Edwards J.A. and Hoke J.E. (1975) Red cell iron uptake in hereditary microcytic anemia. Blood 46, 381-388.
- Morgan E.H. and Bowen B.J. (1986) Anemia of the Belgrade rat: evidence for defective membrane transport of iron. Blood 70, 38-44.
- Oates P.S. and Morgan E.H. (1996) Defective iron uptake by the duodenum of Belgrade rats fed diets of different iron contents Am. J Physiol. 270, G5826-32.
- Su M.A., Trenor C.C., Fleming J.C., Fleming M.D. and Andrews N.C. (1998) The G185R mutation disrupts function of the iron transporter Nramp2. Blood 92, 2157-2163.
- Vidal S.M., Malio D., Vogan K., Skamene E. and Gros P. (1993) Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73, 469-485.
- Oates P.S. and Morgan E.H. (1996) Effects of dietary iron loading with carbonyl iron, and iron depletion on intestinal growth, morphology and expression of the transferrin receptor in the rat. Anat. Rec. 246, 364-371.
- Trinder D. and Morgan E.H. (1997) Inhibition of uptake of transferrin-bound iron by human hepatoma cells by nontransferrin-bound iron. Hepatology 26, 691-698.
| Discussion Board | Previous Page | Your Symposium |