Invited Symposium: Iron Transport
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Iron is an essential element in all mammalian cells as its redox properties facilitate a number of enzyme-catalyzed redox reactions including energy production. At the same time the ability of iron to catalyze formation of potentially toxic reactive oxygen intermediates underscores the importance of homeostatic mechanisms that modulate intracellular iron concentrations. These processes are subserved by transport mechanisms that utilize transferrin-bound or non-transferrin-bound iron (NTBI). Transferrin receptor-mediated endocytosis is relatively well characterized while potential carriers for NTBI have only recently been identified [1, 2]. Our previous studies indicated that NTBI is taken up by human hepatocellular carcinoma (HepG2)cells [3, 4] and rat neonatal cardiac myocytes [5] by a specific, saturable, and temperature-sensitive carrier-mediated process. The fact that such a transport system appears to function in a wide variety of cells [4, 6-10] suggests an important role for this carrier in cellular iron homeostasis. This suggestion was supported by our previous demonstration that NTBI uptake was increased in iron-loaded cells and that this increase was reversed by chelation [3, 5]. No regulatory mechanism to explain these iron-dependent changes in NTBI uptake has emerged as yet.
It is our hypothesis that NTBI uptake may be controlled by intracellular iron-sensing mechanisms that are a well established means of controlling the expression of genes for transferrin-dependent iron transport (transferrin receptor), metabolism (aminolevulinate synthase), and storage (ferritin) [11]. This mechanism requires iron response elements (IRE) in mRNA that are targeted by iron response element-binding proteins (IRP-1 and IRP-2). It has also been suggested that the generation of reactive oxygen species by Fenton-active iron complexes could provide alternative pathways to modulate NTBI transport [12, 13]. One such alternative would be to compromise the permeability barrier through formation of destructive hydroperoxide derivatives of essential plasma membrane lipids and proteins, and thereby increase iron uptake by diffusion. The purpose of this study was to define more clearly how NTBI transport is regulated in mammalian cells.
Materials and Methods
Cell Cultures: HepG2 cells (ATCC HB 8065, Rockville, MD) were grown as described previously [3] in alpha-minimal essential medium (alpha-MEM), supplemented with 10% (v/v) fetal bovine serum (CanSera, Rexdale, Canada), 100 U/ml penicillin, 100 µg/ml streptomycin and non-essential amino acids in a humidified atmosphere of 95% air/5% CO2 at 37°C. COS-7 cells were grown in Dulbecco's Minimal Essential Medium (DMEM), supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin in a humidified atmosphere of 95% air/ 5% CO2 at 37°C. Iron loading was achieved by growing cultures for 1 - 7 days in media supplemented with appropriate ferric ammonium citrate (FAC, 13.75% Fe by mass) concentrations.
Indices of Toxicity: Release of lactate dehydrogenase (LDH) was determined in media after 24 h. Cells were scraped with a rubber policeman into ice-cold phosphate buffered saline (PBS), lysed by sonication, and a cell-free homogenate prepared by centrifugation at 11,500 x g for 5 min. LDH in the medium and in cell homogenates was measured on a Hitachi Auto Analyzer employing the coupled pyruvate/lactate:NADH/NAD reagents from Boehringer Mannheim. Release of LDH into the medium was calculated as a fraction of total cellular LDH and expressed as a percent of cultures with no iron supplementation. Viability was determined with Trypan blue and is the fraction of total cells that exclude the dye. The results are expressed as a percentage of cultures grown in the absence of added iron. The ability of HepG2 cells to synthesize, assemble and secrete two lipoproteins characterized by their apo-lipoprotein moieties apo A-1 (nascent high density lipoprotein) and apo-B (very low density lipoprotein) is one manifestation of the mature hepatocyte phenotype exhibited by these cells. Cultures exposed to various iron concentrations were washed twice with PBS and maintained for 4 h at 37°C in the presence of serum-free medium. The medium was collected, centrifuged at 200 x g for 10 min to remove cell debris, and apo A-1 and apo B concentrations were determined in both cells and medium by enzyme-linked immunosorbent assay. The results are expressed as a percent of cultures with no iron supplementation.
NTBI Uptake: For uptake studies iron in its trivalent oxidized form was stabilized by the addition of a 2.5 molar excess of nitrilotriacetate (NTA) to represent low molecular weight exchangeable ligands such as citrate that exist in plasma. Stock solutions of 0.25 mM [59Fe]Fe/NTA were freshly prepared by mixing alpha-MEM or DMEM, containing 20 mM N-[2-hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid] (HEPES) with the appropriate amounts of [59Fe]FeCl3 (35 mCi/mg; New England Nuclear, Boston, MA), unlabelled 1 mM FeCl3 and 2.5 mM NTA to achieve an Fe/NTA molar ratio of 1:2.5. Before use 0.25 mM [59Fe]Fe/NTA was diluted to 2.5 µM with either alpha-MEM/HEPES or DMEM/HEPES to give a specific radioactivity of 200-600 cpm/pmol Fe. Typically, NTBI transport was measured in 24-well dishes by first washing twice with PBS and incubating in serum-free medium for 60 min to ensure that any potential interference from ferritin, transferrin, and NTBI was minimized. Following an additional wash in serum-free medium 0.25 mL [59Fe]Fe/NTA was added to each well in triplicate and incubated for 15 min at 37°C. Uptake was stopped by the addition of ice-cold PBS to each well, followed by two additional washes in ice-cold PBS. Monolayers were subsequently dissolved in 1% (w/v) sodium dodecyl sulfate and cell-associated 59Fe was measured by gamma counting; protein was determined in the same solution by the method described by Lowry et al. [14]. Counts measured at time zero were subtracted and uptake expressed as fmol/min/µg protein or as a percentage of the uptake in control cells receiving no added iron.
Antisense Transfection: Antisense 21-mer oligonucleotides directed at a position 15 nucleotides upstream from the initiation codon and spanning the first 6 nucleotides of the open reading frame of human IRP-1 [15] were phosphorothioated at each position. COS-7 cells were transfected in 24-well dishes with 3.5 &micr;M antisense oligonucleotide utilizing 2.5 µL/well LipoTaxi (Stratagene, LaJolla, CA) according to the manufacturer's protocol. Briefly, 80 µL of LipoTaxi/oligonucleotide mixtures were added to each well and incubated in serum-free and antibiotic-free DMEM for 5-6 h and then 170 µL of DMEM, supplemented with 20% fetal bovine serum was added and the cells incubated overnight. The next day the transfection solutions were removed and the monolayers washed with PBS. The cultures were then grown in regular growth medium with or without added FAC before NTBI uptake was measured.
Results
Iron loading of HepG2 cells resulted in an increased rate of NTBI uptake and this increase correlated with increased intracellular iron concentrations [3]. That this increase in NTBI transport was not due to compromised plasma membrane permeability was supported by the following three observations. First, lactate dehydrogenase in the growth medium is a measure of the cell integrity since the permeability of the plasma membrane would prevent the loss of this cytoplasmic enzyme. Exposure of HepG2 cells to 5 to 80 µg Fe/ml in the medium for 7 days results in significant increases in intracellular iron concentrations [3]. However, there is no significant leakage of LDH under these conditions(Fig.1).
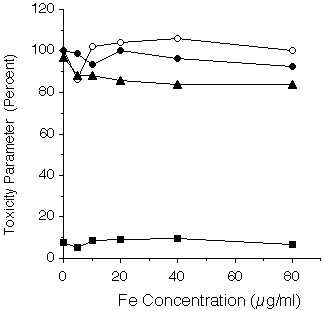 Fig. 1: Effect of iron loading upon indices of toxicity in HepG2 cells. Secreted apo A-1 (open circles) and apo-B (closed circles) are expressed as a percentage of control cells receiving no iron. LDH leakage was measured in the growth medium as a percentage of total cellular LDH (squares). Viability (triangles) was determined by Trypan blue dye exclusion and expressed as a percentage of control cells.
Fig. 1: Effect of iron loading upon indices of toxicity in HepG2 cells. Secreted apo A-1 (open circles) and apo-B (closed circles) are expressed as a percentage of control cells receiving no iron. LDH leakage was measured in the growth medium as a percentage of total cellular LDH (squares). Viability (triangles) was determined by Trypan blue dye exclusion and expressed as a percentage of control cells.
Viability, determined by Trypan Blue dye exclusion, was also unaffected under these conditions in keeping with an intact plasma membrane permeability barrier. Secondly, the ability of HepG2 cells to coordinate the synthesis, assembly and secretion of two different classes of lipoprotein is an independent measure of HepG2 viability. These major biosynthetic mechanisms are also unaffected by iron loading in that there is no relation between extracellular levels of either apo A-1 or apo B apo-lipoproteins and medium iron concentration (Fig. 1). Thirdly, the efflux of radiolabeled iron was measured over a 3 h time interval in the presence of various media iron concentrations from cells exposed to 20µg Fe/ml for 24h (Table 1).
Extracellular [Fe] (µM) Fe Efflux (%) -------------------------------------------------- 0 11.9 ± 0.8 1 12.6 ± 1.1 10 11.4 ± 0.2 100 11.7 ± 0.5 --------------------------------------------------In the absence of any exogenous iron 11.9 ± 0.8% was released and this rate of efflux was unaffected by the presence of 1 - 100 µM exogenous iron concentrations. Indeed, efflux of iron only increased when 1 mM deferiprone (39%) or desferrioxamine (40%) were present in the medium, indicating that iron loading per se did not compromise the permeability of the plasma membrane. Taken together these results indicate that iron loading has no adverse affects upon either viability or permeability of HepG2 cells but rather suggests that the up-regulation of NTBI uptake by iron loading is coupled to intracellular iron-sensing mechanisms.
Our hypothesis is that such a system may be responsible in part for the increase in NTBI transport in response to elevated intracellular iron concentrations. To test this, IRP-1 synthesis was suppressed by transfecting COS-7 cells with an antisense oligonucleotide directed toward the translation initiation site of IRP-1 mRNA. As seen in Fig.2
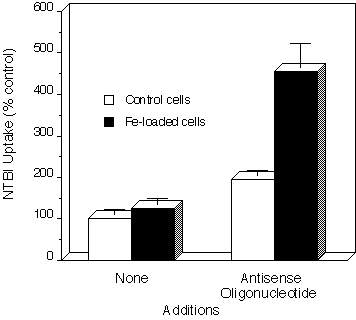 Fig. 2: The effect of antisense oligonucleotide to IRP-1 on the uptake of NTBI in control and iron-loaded COS-7 cells. COS-7 cells were
transfected with 3.5 µM oligonucleotide and incubated for 24 h in the
presence (open bars) or absence (closed bars) of 20 µg Fe/ml. NTBI
uptake was measured in triplicate wells and expressed as a percent (mean
± SD) of control, untreated cells.
Fig. 2: The effect of antisense oligonucleotide to IRP-1 on the uptake of NTBI in control and iron-loaded COS-7 cells. COS-7 cells were
transfected with 3.5 µM oligonucleotide and incubated for 24 h in the
presence (open bars) or absence (closed bars) of 20 µg Fe/ml. NTBI
uptake was measured in triplicate wells and expressed as a percent (mean
± SD) of control, untreated cells.
The antisense oligonucleotide increased NTBI uptake nearly two-fold (196%)in cells grown in the absence of any added iron (control cells). Control cells grown in the presence of 20 µg Fe/ml for 24 h showed a modest increase (126%) in iron uptake, consistent with our previous observations in other cell types. However, similar iron loading of antisense-treated cells increased NTBI uptake over four-fold (455%).
Discussion and Conclusion
The role of iron-catalyzed free radical production in NTBI uptake is controversial. On the one hand there are reports that hydroxyl radicals generated by the Haber-Weiss reaction stimulate NTBI uptake [16] while other findings appear not to support such a mechanism [17]. The findings in the present report appear also to be inconsistent with a major role of iron-catalyzed free radical production in the up-regulation of NTBI transport. While the presence of end-point reaction products (e.g., malondialdehyde or hydroxynonenal) may exist in measurable concentrations in iron overload, the relative contribution of compensatory defense/repair mechanisms (free radical scavengers; antioxidants; or enzymes such as catalase, glutathione peroxidase, or superoxide dismutase) remains to be assessed. The failure of iron loading to alter several indices of viability and permeability in HepG2 cells suggests these defense mechanisms are adequate to meet the demands imposed by high intracellular iron. Indeed, increased ferritin production and therefore enhanced storage occurs in these cells [18, 19], suggesting that the liver can sequester iron as an additional means to detoxify the iron-loaded intracellular milieu. Notwithstanding these protective measures, evidence that iron may exacerbate hepatotoxic conditions such as fibrosis and alcoholic cirrhosis persist [20] but the mechanisms remain to be delineated.
Candidates for mammalian iron transport proteins have only recently been reported and include SFT [1], possibly responsible for NTBI transport as well as intracellular transferrin-bound iron transfer. This carrier may be coupled to a copper-dependent ferrireductase [21]. Another protein, DCT1/Nramp2 [2], may function as a generalized divalent cation carrier.
However, Nramp2 is up-regulated by iron depletion in contrast to NTBI uptake in our studies. Nramp2 mRNA contains an IRE in the 3' untranslated region that confers iron-dependent stabilization [22]. In contrast, the present finding that an antisense oligonucleotide directed toward IRP-1 enhanced NTBI uptake is in keeping with a transcript having an IRE on the 5' side, since decreased IRP-1 would allow for greater NTBI carrier synthesis in a manner analogous to ferritin post-transcriptional regulation. The enhancing effect of iron loading upon iron uptake would also be consistent with this interpretation in that reconstitution of the IRP-1 [4Fe-4S] cofactor would promote dissociation from IRE. The finding that there was further enhancement of iron uptake after iron loading may also be an indication of additional stimulatory effects possibly mediated through interactions with upstream translation-enhancing sites analogous to those reported for ferritin [23].
References
1. Gutierrez, J., Yu, J.M., Rivera, S. and Wessling-Resnick, M., Functional expression cloning and characterization of SFT, a stimulator of Fe transport. J. Cell Biol. 139: 895-905 (1996).
2. Gunshin, H., Mackenzie, B., Berger, U., Gunshin, Y., Romero, M., Boron, W., Nussberger, S., Gollan, J. and Hediger, M., Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482-488 (1997).
3. Parkes, J., Randell, E., Olivieri, N. and Templeton, D., Modulation by iron loading and chelation of the uptake of non-transferrin-bound iron by human liver cells. Biochi. Biophys. Acta 1243: 373-380 (1995).
4. Randell, E.W., Parkes, J.G., Olivieri, N.F. and Templeton, D.M., Uptake of non-transferrin-bound iron by both reductive and nonreductive processes is modulated by intracellular iron. J. Biol. Chem 269: 16046-16053 (1994).
5. Parkes, J.G., Hussain, R.A., Olivieri, N.F. and Templeton, D.M., Effects of iron loading on uptake,speciation and chelation of iron in cultured myocardial cells. J. Lab Clin Med 122: 36-47 (1993).
6. Sturrock, A., Alexander, J., Lamb, J., Craven, C.M. and Kaplan, J., Characterization of a transferrin-independent uptake system for iron in HeLa cells. J. Biol. Chem. 265: 3139-3145 (1990).
7. Barisani, D., Berg, C.L., Wessling-Resnick, M. and Gollan, J.L., Evidence for a low Km transporter for non-transferrin-bound iron in isolated rat hepatocytes. Am. J. Physiol. 269: G570-G576 (1995).
8. Inman, R.S. and Wessling-Resnick, M., Characterization of transferrin-independent iron transport in K562 cells. Unique properties provide evidence for multiple pathways of iron uptake. J. Biol. Chem. 268: 8521-8528 (1993).
9. Morgan, E.H., Membrane transport of non-transferrin-bound iron by reticulocytes. Biochim. Biophys. Acta 943: 428-439 (1988).
10. Wright, T.L., Brissot, P., Ma, W.-L. and Weisiger, R.A., Characterization of non-transferrin-bound iron clearance by rat liver. J. Biol. Chem. 261: 10909-10914 (1986).
11. Hentze, M.W. and Kühn, L.C., Molecular control of vertebrate iron metabolism: MrNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc. Natl. Acad. Sci. USA 93: 8175-8182 (1996).
12. Bacon, B.R. and Britton, R.S., The pathology of hepatic iron overload: a free radical-mediated process? Hepatology 11: 127-137 (1990).
13. Halliwell, B. and Gutteridge, J.M.C., Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 307: 108-112 (1992).
14. Lowry, O., Rosebrough, N., Farr, A. and Randall, R., Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275 (1951).
15. Hirling, H., Emery-Goodman, A., Thompson, N., Neupert, B., Seiser, C. and Kühn, L.C., Expression of active iron regulatory factor from a full-length human cDNA by in vitro transcription/translation. Nucleic Acids Research 20: 33-39 (1992).
16. Richardson, D.R. and Ponka, P., Identification of a mechanism of iron uptake by cells which is stimulated by hydroxyl radicals generated via the iron-catalysed Haber-Weiss reaction. Biochim. Biophys. Acta 1269: 105-114 (1995).
17. Qian, Z.M., Tang, P.L. and Morgan, E.H., Effect of lipid peroxidation on transferrin-free iron uptake by rabbit reticulocytes. Biochim. Biophys. Acta 1310: 293-302 (1996).
18. Lescoat, G., Loreal, O., Moirand, R., Pasdeloup, N., Deugnier, Y. and Brissot, P., Iron induction of ferritin synthesis and secretion in human hepatoma cell (Hep G2) cultures. Liver 179-185 (1989).
19. Lescoat, G., Hubert, N., Moirand, R., Jego, P., Pasdeloup, N. and Brissot, P., Iron load increases ferritin synthesis and secretion in adult human hepatocyte cultures. Liver 11: 24-29 (1991).
20. Pietrangelo, A., Metals, oxidative stress, and hepatic fibrogenesis. Seminars Liver Dis. 16: 13-30 (1996).
21. Yu, J. and Wessling-Resnick, M., Influence of copper depletion on iron uptake mediated by SFT, a stimulator of Fe transport. J. Biol. Chem. 273: 6909-6915 (1998).
22. Lee, P.L., Gelbart, T., West, C., Halloran, C. and Beutler, E., The human Nramp2 gene: Characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol. Dis. 24: 199-215 (1998).
23. Dix, D., Lin, P.-N., Kimata, Y. and Theil, E., The iron regulatory region of ferritin mRNA is also a positive contol element for iron-independent translation. Biochemistry 31: 2818-2822 (1992).
| Discussion Board | Previous Page | Your Symposium |