Invited Symposium: Role of the Basal Forebrain Neurons in Cortical Activation and Behavioural State Regulation
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Adenosine (AD) has been implicated as an endogenous sleep factor (Radulovacki et al., 1984; Rainnie et al., 1994; Bennington and Heller, 1995). Microinjection into the preoptic area of rats (Ticho and Radulovacki, 1991), and microdialysis into the LDT/PPT and basal forebrain (Portas et al., 1996, 1997) in cats, produce significant increases in sleep. Endogenous AD increases with sleep deprivation and decreases during sleep in the basal forebrain of cats (Porka-Heiskanen et al., 1997). In addition, basal forebrain perfusion of nitrobenzylthioionosine, an AD transport blocker, caused increased extracellular AD in conjunction with increased sleep (Porka-Heiskanen et al., 1997). In vitro, AD inhibits LDT/PPT cholinergic neurons and basal forebrain neurons (Rainnie et al., 1994). These data suggest AD may effect behavioral state by inhibiting arousal systems.
The preoptic/anterior region of the hypothalamus (POAH) has been hypothesized to be an important somnogenic center in the CNS (von Economo, 1930; Nauta, 1946; McGinty and Szymusiak, 1990). Lesions of the POAH in rats (Nauta, 1946; Szymusiak and Satinoff, 1984; John et al., 1994) and cats (McGinty and Sterman, 1968; Sallanon et al.,1989; Szymusiak et al., 1991) produce significant reductions in sleep. Microinjection of somnogens such as AD (Ticho and Radulovacki, 1991), triazolam (Mendelson et al., 1989), and prostaglandin D2 (Ueno et al., 1983) into the POAH all significantly increase sleep. In addition, the POAH contains a population of sleep-active neurons whose firing rates are lowest during active waking and highest during NREM sleep (Findlay and Hayward, 1969; Hayaishi and Osaka, 1995; Koyama and Hayaishi, 1994; Kaitin, 1984; Szymusiak and McGinty, 1986; Szymusiak and McGinty, 1989; Alam et al., 1995; Alam et al., 1996). These neurons comprise about 25% of the cells seen in vivo . They are dispersed throughout a heterogeneous population of cells, and the neurotransmitter content of these sleep-active neurons is not known. Consequently, the electrophysiological and chemical properties of these neurons had not been specifically studied.
Recently, a cluster of neurons in the ventrolateral preoptic area of the hypothalamus (VLPO) have been found to express c-fos in association with sleep (Sherin et al., 1996). Expression of c-fos is often associated with increased neuronal activity. Therefore, the VLPO has been hypothesized to contain a cluster of sleep-active neurons. Preliminary results in vivo support this hypothesis (McGinty et al., 1997 abst).
This clustering of c-fos positive and sleep-active neurons in the VLPO has facilitated studies of the electrophysiological properties of these neurons and their response to AD application. If AD is an important endogenous sleep factor and if the VLPO sleep-active neurons are an important part of the sleep regulatory pathway then we would predict that AD should excite these VLPO neurons. However, in the CNS AD is generally regarded as an inhibitory neuromodulator, primarily through activation of A1 receptors. Nonetheless, A2 receptor activation is linked to increased intracellular cAMP concentrations (Dunwiddie, 1985; Olah and Stiles, 1995) so a direct excitatory effect remains a possibility. In addition, AD has recently been reported to inhibit the release of GABA in SCN and arcuate nucleus cell cultures (Chen and van den Pol, 1997). Thus, a disinhibition of VLPO neurons by AD may also be a possible mechanism for sleep promotion. The present report investigates these hypotheses using whole-cell patch clamp techniques in rat brain slices.
Materials and Methods
Six to eight week old (200-300g) hooded Long Evans rats were anesthetized and decapitated. The brains were rapidly removed and placed in ice cold artificial cerebral spinal fluid (aCSF) bubbled with 95% O2/5% CO2. Horizontal sections (400 u m) were cut on a vibrating tissue slicer (model OTS 3000, Electron Microscopy Instruments) and incubated at room temperature for one hour. A slice containing the VLPO was placed in a recording chamber and profused with aCSF at a rate of 1.5 ml/min. The temperature of the aCSF bathing the slice was gradually raised to 36.5 (+/- 0.5) oC. aCSF contained (in mM) NaCl 124, KCl 2, KH2PO4 3, MgCl2 1.3, CaCl2 2.5, glucose 10, and NaHCO3 26. The patch electrode solution contained (in mM) K-Gluconate 120, KCl 10, MgCl2, 3, HEPES 10, K2 ATP 2, Na2 GTP 0.2, 0.25% biocytin, and pH adjusted to 7.2 with 1 N KOH.
Whole cell patch-clamp recordings were made using an Axopatch 1D amplifier (Axon Instruments). Patch pipettes were pulled on a Flamming/Brown horizontal puller (model P97, Sutter Instruments) to a resistence of 6-10 MOhms. Data was collected and analyzed using pClamp 6.2 software (Axon Instruments). Adenosine, bicuculline and DNQX were bath applied.
Results
From stable recordings of 78 units, we found the VLPO to be an electrophysiologically heterogenous population of neurons. Fifteen percent displayed burst firing in response to depolarizing current steps. Non-bursting neurons displayed heterogenous responses to depolarizing current steps including rhythmic firing, spike frequency adaptation, or accommodation. The burst capable neurons displayed lower input resistance than non-bursting neurons (522.3 +/- 40.9 vs. 659.6 +/- 68.4 MOhms). Most neurons (90%) displayed Ih-like currents (voltage-gated inward rectifying K+-current) and /or IT-like currents (low threshold Ca++-current).
 click to enlarge
Figure 1: Voltage-clamp recordings of two neurons using voltage-ramp protocols. One cell (A) displays predominantly ipsc's that are blocked by 30 uM bicuculline (C). The second cell (B) displays a mix of ipsc's and epsc's. The epsc's are blocked by 40 u M DNQX (D).
click to enlarge
Figure 1: Voltage-clamp recordings of two neurons using voltage-ramp protocols. One cell (A) displays predominantly ipsc's that are blocked by 30 uM bicuculline (C). The second cell (B) displays a mix of ipsc's and epsc's. The epsc's are blocked by 40 u M DNQX (D).
Spontaneous synaptic activity was pronounced in most recorded neurons and consisted of either fast epsp/c's or fast ipsp/c's. Non-bursting neurons displayed higher rates of spontaneous events than bursting neurons. Spontaneous events for non-bursting neurons were either primarily ipsp/c's or mixed ipsp/c's and epsp/c's. The low level spontaneous events of the bursting neurons were primarily epsp/c's. The ipsp/c's were blocked by 30 uM bicuculline suggesting they are GABAA mediated events (three of three units tested, figure 1). The epsp/c's were blocked by 40 uM DNQX suggesting they are mediated by the AMPA subtype of glutamate receptors (three of three units tested).
 click to enlarge
Figure 2: Voltage-clamp recordings from one neuron under baseline (A), 30 uM adenosine (B), and recovery (C). Adenosine blocked the ipsc's selectively (B).
click to enlarge
Figure 2: Voltage-clamp recordings from one neuron under baseline (A), 30 uM adenosine (B), and recovery (C). Adenosine blocked the ipsc's selectively (B).
AD (20-100 uM) differentially effected the spontaneous synaptic events on bursting and non-bursting neurons. In 10 of 15 non-bursting neurons, AD decreased the number of ipsp/c's by 47% to 100% (figure 2), the epsp/c's were generally unaffected. Conversely, in two of four bursting neurons, AD decreased the number of epsp/c's by 64% and 70%. There was no effect in the remaining two.
Discussion and Conclusion
AD disinhibits a significant population of VLPO neurons by decreasing spontaneous ipsp/c's in vitro. By decreasing a tonic inhibitory tone, AD may allow for increases in neuronal activity. We found this to be the case in two neurons that we studied in both current clamp and voltage clamp conditions. One neuron was not spontaneously active under baseline conditions but became active with application of 50 uM AD. The other neuron's spontaneous activity was increased almost two-fold with 100 uM AD. Activity returned to baseline levels after washout. If the sleep-active neurons of the VLPO are an important part of a somnogenic pathway, then AD may help facilitate sleep by disinhibiting these neurons. Although there is not a direct excitatory effect on membrane potential, increasing AD levels during waking may help activate the VLPO sleep-active neurons by removing GABAergic inhibitory inputs. Figure 1 shows a schematic of this hypothesis.
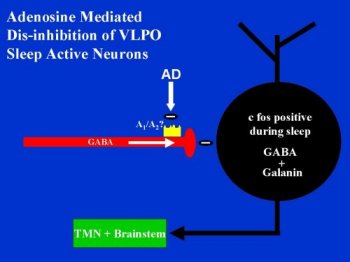 click to enlarge
Figure 3: A schematic drawing of our working hypothesis of how adenosine may affect the sleep-active neurons of the VLPO.
click to enlarge
Figure 3: A schematic drawing of our working hypothesis of how adenosine may affect the sleep-active neurons of the VLPO.
AD may be contributing to the regulation of behavioral state on two ways. First, AD may be directly inhibiting arousal systems such as the LDT/PPT and basal forebrain cholinergic systems. Secondly, AD may promote sleep by blocking inhibitory inputs onto sleep-active neurons. The first mechanism has support both in vivo (Portas et al., 1996, 1997; Porka-Heiskanen et al., 1997) and in vitro (Rainnie et al., 1994). The current study would support the second mechanism if the population of neurons that are disinhibited by AD could be shown to correspond to the population of VLPO sleep-active neurons. The c-fos positive VLPO neurons display an 80% co-localization with the neuropeptide galanin (Cliff Saper, personal communication). Currently we are investigating double labeling of our biocytin filled neurons using in situ hybridization galanin mRNA.
References
Alam, M.N., McGinty, D., and Szymusiak, R. (1995) Neuronal discharge of preoptic anterior hypothalamic thermoresponsive neurons: relation to nonrapid eye movement sleep. Am. J. Physiol. 269:1250-1257.
Alam, M.N., McGinty, D., and Szymusiak, R. (1996) Lateral preoptic area and diagonal band thermosensitive neurons in rats: relation to wakefulness and non rapid eye movement sleep. Brain Research 718:76-82
Benington, J.H. and Heller, H.C. (1995) Restoration of brain energy metabolism as the function of sleep. Progress in Neurobiology 45:347-360.
Chen, G. and Van den Pol, A.N. (1997) Adenosine modulation of calcium currents and presynaptic inhibition of GABA release in suprachiasmatic and arcuate nucleus neurons. J. Neurophysiol. 77:3035-3047.
Dunwiddie, T.V. (1985) The physiological role of adenosine in the central nervous system. Int. Rev. Neurobiol. 27:63-139.
Findlay, A.R. and Hayward, J.N. (1969) Spontaneous activity of single neurones in the hypothalamus of rabbits during sleep and waking. J. Physiol. 201:237-258.
John, J., Kumar, V.M., Gopinath, G., Ramesh, V., and Mallick, H. (1994) Changes in sleep-wakefulness after kainic acid lesion of the preoptic area in rats. Japanese Journal of Physiology 44:231-242.
Kaitin, K.I. (1984) Preoptic area unit activity during sleep and wakefulness in the cat. Exp. Neurol. 83:347-357.
Koyama, Y. and Hayaishi, O. (1994) Modulation by prostaglandins of activity of sleep-related neurons in the preoptic/anterior hypothalamic areas of rats. Brain Res. Bull. 33:367-372.
McGinty, D.J., Alam, M.N., and Szymusiak, R. (1997) Sleep deprivation increases the sleep-related discharge of sleep-active ventrolateral preoptic neurons. Society for Neuroscience Abstracts 23.
McGinty, D.J. and Sterman, M.B. (1968) Sleep suppression after basal forebrain lesions in the cat. Science 160:1253-1255.
McGinty, D.J. and Szymusiak, R. (1990) Keeping cool: a hypothesis about the mechanisms and functions of slow wave sleep. Trends Neurosci. 13:480-487.
Mendelson, W.B., Martin, J.V., Perlis, M., and Wagner, R. (1989) Enhancement of sleep by microinjection of triazolam into the medical preoptic area. Neuropsychopharm. 2:61-66.
Nauta, W.J.H. (1946) Hypothalamic regulation of sleep in rats. An experimental study. J. Neurophysiol. 9:285-316.
Olah, M.E. and Stiles, G.L. (1995) Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 35:581-606.
Porkka-Heiskanen, T., Strecker, R.E., Thakkar, M., Bjørkum, A.A., Greene, R.W., and McCarley, R.W. (1997) Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276:1265-1268.
Portas, C.M., Thakkar, M., Rainnie, D.G., Greene, R.W., and McCarley, R.W. (1997) Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience 79:225-235.
Portas, C.M., Thakkar, M., Rainnie, D.G., and McCarley, R.W. (1996) Microdialysis perfusion of 8-OH-DPAT in the dorsal raphe nucleus decreases serotonin release and increases REM sleep in freely moving cat. J. Neurosci. 16:2820-2828.
Radulovacki, M., Virus, R.M., Djuricic-Nedelson, M., and Green, R.D. (1984) Adenosine analogs and sleep in rats. J. Pharmac. Exp. Ther. 228:268-274.
Rainnie, D.G., Grunze, H.C., McCarley, R.W., and Greene, R.W. (1994) Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science 263:689-692.
Sallanon, M., Sakai, K., Denoyer, M., and Jouvet, M. (1989) Long lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus. J. Neurosci. 32: 669-683.
Sherin, J.E., Shiromani, P.J., McCarley, R.W., and Saper, C.B. (1996). Activation of ventrolateral preoptic neurons during sleep. Science 271:216-219.
Szymusiak, R., Danowski, J. and McGinty, D. (1991) Exposure to heat restores sleep in cats with preoptic/anterior hypothalamic cell loss. Brain Res. 541:134-138.
Szymusiak, R. and McGinty, D. (1986) Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 370:82-92.
Szymusiak, R. and McGinty, D. (1989) Sleep-waking discharge of basal forebrain projection neurons in cats. Brain Res. Bull. 22:423-430.
Szymusiak, R. and Satinoff, E. (1984) Ambient temperature-dependence of sleep disturbances produced by basal forebrain damage in rats. Brain Res. Bull. 12:295-305.
Ticho, S.R. and Radulovacki, M. (1991) Role of adenosine in sleep and temperature regulation in the preoptic area of rats. Pharmacol. Biochem. Behav. 40:33-40.
Ueno, R., Honda, K., Inoue, S., and Hayaishi, O. (1983) Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc. Natl. Acad. Sci. USA 80:1735-1737.
Von Economo, C. (1930) Sleep as a problem of localization. J. Nerv. Ment. Dis. 71:249-259.
| Discussion Board | Previous Page | Your Symposium |