Invited Symposium: Perspectives on Behavioural Function of Dopamine in the Nucleus Accumbens
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Effects of Accumbens DA Depletions on Lever Pressing
The purpose of this review is not to provide a broad survey of all areas of research on nucleus accumbens; such a task would require a large monograph, and even then it would be incomplete. Rather, this article is intended to provide a summary of the research on the behavioral effect of accumbens dopamine (DA) depletions. Specifically, the pages below will focus on the effects of accumbens DA depletions on various food-related instrumental tasks. This work was undertaken partly because of the hypothesized involvement of accumbens DA in "reward" or "reinforcement" processes.
Although the vast majority of studies on the purported "reward" functions of DA have focussed upon self-administration and the effects of drugs of abuse, it should be emphasized that a critical part of the DA/reward hypothesis is that accumbens DA mediates the reinforcing properties of natural stimuli, such as sex and food (Hernandez and Hoebel, 1988; Smith, 1995; Wise, 1982, 1985). Indeed, according to what has been described as the General Anhedonia Model (see Salamone et al., 1997), a major tenet of the DA/reward hypothesis is that a natural reward system evolved to mediate reinforcement processes with regards to natural reinforcers, and that this same system is activated by drugs of abuse.
Perhaps the most salient feature of the literature on the effects of accumbens DA depletions on lever pressing is that the results of the depletion depend greatly upon the particular schedule of reinforcement being used. In fact, on many schedules, the most obvious thing to report is that substantial DA depletions have little effect on operant responding at all. In two studies, the effects of accumbens DA depletions on continuously reinforced responding were assessed. The major effect of accumbens DA depletions was an initial slowing of responding during the first few minutes of the test session, which was only evident for a few days of postsurgical testing (McCullough et al., 1993a; Salamone et al., 1995).
Another result of the DA depletions was a slowing of the distribution of interresponse times (IRTs; Salamone et al., 1995); this particular result will be discussed in greater detail below. Yet despite the effects on response patterning that were observed, it should be emphasized that global measures of response output on the continuous schedule were basically unaffected by accumbens DA depletions (see also Aberman and Salamone, submitted). These findings are important in view of the fact that this schedule represents the most fundamental schedule of primary, positive reinforcement.
Interval schedules also have been investigated. Sokolowski and Salamone (1998) injected 6-hydroxydopamine (6-OHDA) into either the core or shell of the nucleus accumbens in animals trained to respond on a variable interval 30-sec (VI30) schedule. Neither core nor shell DA depletions had any significant effect upon VI30 lever pressing. Another recent study compared the effects of nucleus accumbens DA depletions with those of DA depletions in ventrolateral striatum (VLS). In that study, responding on a fixed interval 30-sec (FI30) schedule was substantially affected by VLS DA depletions, while accumbens DA depletions had only a modest suppressive effect on lever pressing (Cousins et al., in press). Although rats with accumbens DA depletions exhibited lever pressing levels that were significantly lower than those shown by control animals, rats with accumbens DA depletions demonstrated significantly higher levels of responding than rats with VLS DA depletions.
Several recent experiments have used ratio schedules to investigate the effects of accumbens DA depletions. In another study that compared the effects of accumbens and VLS DA depletions, a fixed ratio 5 (FR5) schedule was employed (Salamone et al., 1993b). As with the experiment reviewed above, VLS DA depletions produced severe impairments in FR5 lever pressing. Accumbens DA depletions did significantly reduce FR5 lever pressing compared to controls, although rats with accumbens depletions had significantly higher levels of responding than rats with VLS depletions. In addition, the deficit in total number of responses shown by rats with accumbens DA depletions was only significant during the first week of post surgical testing. Similar to what was shown with the continuous schedule, analysis of the IRT distributions showed that accumbens DA depletions produced a slowing of the local rate of responding. Sokolowski and Salamone (1998) also used the FR5 schedule to study the effects of core and shell injections of 6-OHDA.
Although shell injections of 6-OHDA had no significant effects on FR5 responding, injections into the accumbens core site suppressed FR5 responding and altered the IRT distribution. In a recent study (Aberman and Salamone, submitted), the effects of accumbens DA depletions were assessed using four schedules: FR1, FR4, FR16, and FR64. Rats with accumbens DA depletions showed behavioral deficits that were highly dependent upon the FR value; there were no effects of DA depletions on FR1 lever pressing, but with larger ratio values, the impairment was much greater. Accumbens DA depletions appear to be facilitating an effect known as "ratio strain". Normally, response rates are higher at larger FR values than at smaller FR values. At some point, the ratio value is too large, and rats decrease responding (Staddon and Ettenger, 1989). In the Aberman and Salamone (submitted) study, accumbens DA depletions altered the functional relation between ratio value and response rate, effectively increasing the response-suppressing effects of large ratio values.
Another line of investigation has been to study the behavior of rats tested in choice procedures, in which there are alternative paths to reinforcement that involve different instrumental response requirements. The primary behavioral procedure that has been used was one in which rats have a choice of pressing a lever on a FR5 schedule to receive a more preferr-ed food (Bioserve pellets), or simply feeding upon a less preferred food (lab chow) that is freely available in the operant chamber (i.e. concurrent FR5/feeding task; Salamone et al., 1991). Untreated rats will work for the preferred food by lever pressing, and will consume very little of the freely available but less preferred lab chow. Administration of the DA antagonist halo-peridol decreased lever pressing for food but increased lab chow consumption. Haloperidol was shown not to alter food preference in free-feeding choice tests, and the effects of haloperidol did not resemble the effects of pre-feeding to reduce food motivation. DA antagonists with different receptor subtype specificities (i.e., haloperidol, cis-flupenthixol, SCH 23390, SKF 83566) all were shown to decrease lever pressing and increase chow consumption substantially (Cousins et al., 1994; unpublished observations). Considerable research indicated that nucleus accumbens is the critical brain locus at which DA antagonism or DA depletions decrease lever pressing and increase chow consumption in the concurrent FR5/feeding task (Cousins et al., 1993; Cousins and Salamone, 1994; Salamone et al., 1991). Injections of 6-OHDA into the nucleus accumbens core significantly decreased lever pressing for food pellets, increased lab chow consumption, and decreased the relative amount of food obtained by lever pressing (Sokolowski and Salamone, 1998). Dorsomedial shell injections of 6-OHDA had no significant effects on either lever pressing or lab chow consumption. Accumbens DA depletions also produced similar effects in a T-maze version of the cost/benefit procedure. On this task, rats were given a choice between an arm with a 44 cm barrier that contained 4 food pellets and an arm with no barrier that contained 2 food pellets.
Rats with accumbens DA depletions shifted their choice from the arm that has a high barrier and a high density of food reinforcement, and instead chose the arm of the maze with no barrier, but a lower density of food reinforcement (Cousins et al., 1996; Salamone et al., 1994a; for review see Salamone et al., 1997). Thus, rats with accumbens DA depletions showed dramatic shifts away from lever pressing or barrier crossing, and instead selected less preferred or lower density food sources if they had a lower work requirement. The T-maze results also indicate that the DA antagonist- or DA-depletion-induced shift in responding away from FR5 responding in the concurrent FR5/feeding procedure is not merely an artifact of using lever pressing, and does not simply involve a shift away from an "instrumental" response to a "consummatory" response.
It should be stressed that these deficits did not occur because of severe or gross motor deficits, or absolute ceilings on response output. In one study, we compared the effects of accumbens DA depletions on the FR5/chow feeding task with effects on the FR5 schedule alone, without chow present (Cousins and Salamone, 1994). On days when chow was not present, FR5 responding was only mildly affected by accumbens DA depletions; there was no significant overall response deficit in DA-depleted rats, although the more severely depleted rats showed slight reductions in responding. Nevertheless, on alternate days, when chow was available in the chamber during the operant session, DA depleted rats showed a substantial decrease in FR5 lever pressing, and a concomitant increase in chow consumption. Similar results were obtained with the T-maze task. Accumbens DA depletions did not affect the choice of the arm with the barrier if the other arm in the maze did not contain food; the shift in responding only occurred if the arm without the barrier contained food (Cousins et al., 1996).
Accumbens DA Depletions do not Block Primary Reinforcement
As noted above, one of the most popular notions in behavioral neuroscience has been that DA, particularly in nucleus accumbens, mediates the reinforcing impact of stimuli such as food, water, sex and drugs of abuse. Indeed, in a recent review article by Smith (1995) it was claimed that the DA hypothesis of reward had been "proved". Yet despite the popularity of this hypothesis, there are enormous difficulties with the idea that accumbens DA mediates the primary reinforcing characteristics of natural stimuli such as food (see Salamone et al., 1997).
Although it often is stated that accumbens DA depletions affect "reward" because they blunt stimulant self-administration, it should be emphasized that depletions of accumbens DA that severely affected cocaine self-administration had little effect on food reinforced responding (Caine and Koob, 1994; Roberts et al., 1977). Although Smith (1995; see also Schneider et al., 1989, 1990) emphasized that sucrose consumption can be affected by intra-accumbens injections of DA antagonists, there are numerous problems with these studies. Very high doses (i.e., nearly systemic doses) of SCH 23390 and raclopride were injected directly into the accumbens, so the anatomical specificity of the drug injections is questionable. Although Smith (1995) argued that the fact that the local lick frequency is not affected by intracranial DA antagonists proves that there is no motor effect being produced, such an argument is extremely spurious. In fact, the local lick frequency is set by brainstem pattern generators rather than the basal ganglia, and this frequency is not affected by even cataleptogenic doses of DA antagonists (Fowler and Das, 1994). Other motor parameters are affected by systemic and intra-accumbens DA antagonists, including lick efficiency, lick duration, lick force, lap volume and tongue extension (Fowler and Das, 1994; Fowler and Mortell, 1992; Gramling and Fowler, 1985; Hsiao and Smith, 1995; Jones and Mogenson, 1979; Schneider et al., 1990; Schneider et al., 1989).
Fundamental aspects of food reinforcement and food motivation are intact after interference with accumbens DA transmission. Appetitive taste reactivity to sucrose is unaffected by accumbens DA depletions (Berridge, 1996). Accumbens DA depletions did not affect the discrimination of reinforcement magnitude, and failed to alter response selection based upon reinforcement magnitude, in a food-reinforced T-maze task (Cousins et al., 1996; Salamone et al., 1994a). Several lines of evidence indicate that accumbens DA depletions do not generally suppress appetite. It has been reported that accumbens DA depletions or intra-accumbens injections of DA antagonists do not affect food intake (Bakshi and Kelley, 1990; Koob et al., 1978; Salamone et al., 1993a). Accumbens DA depletions have little or no effect upon the total amount of food obtained by FR1 lever pressing (Aberman and Salamone, submitted; McCullough et al., 1993; Salamone et al., 1995); the effects on response patterning that have been reported previously (i.e., initial slowing and slowing of the interresponse time distribution) did not resemble those of extinction (McCullough et al., 1993a; Salamone et al., 1995).
Indeed, the presumed relation between extinction and interference with DA systems appears to be a gross oversimplification; as emphasized in a recent review (Salamone et al., 1997), several articles have shown that, upon closer examination, DA antagonists and DA depletions produce effects that are distinct from those of extinction (Asin and Fibiger, 1984; Ettenberg and Carlisle, 1995; Evenden and Robbins, 1983; Faustman and Fowler, 1981, 1982; Gramling et al., 1984, 1987; Mason et al., 1980; McCullough et al., 1993a; Phillips and Fibiger, 1979; Salamone, 1986; Salamone et al., 1995; Spivak and Amit, 1986; Tombaugh et al., 1980, 1983; Willner et al., 1988).
As noted above, with rats on a concurrent FR5/chow feeding schedule, accumbens DA depletions or intra-accumbens injections of haloperidol decreased lever pressing but increased chow consumption (Cousins et al., 1993; Cousins and Salamone, 1994; Salamone et al., 1991; Sokolowski and Salamone, 1998). Thus, it seems untenable to maintain that accumbens DA depletions suppress lever pressing on some schedules because they produce a general reduction in food motivation. In fact, pre-feeding to reduce food motivation was shown to suppress both lever pressing and chow consumption on the concurrent lever pressing/chow feeding task (Salamone et al., 1991). As previously described, the effects of accumbens DA depletions do not closely resemble the effects of extinction. In this context, it is worth emphasizing that the effects of accumbens DA depletions also bear little resemblance to the effects of pre-feeding. A recent study (Aberman and Salamone, submitted) employed FR schedules with various ratio requirements (FR1, FR4, FR16, FR64), and it was observed that the effects of accumbens DA depletions were determined by the schedule; there was no significant effect of accumbens DA depletions on FR1 responding, and as ratio value increased, accumbens DA depletions caused greater suppression of responding.
A second experiment studied the effects of pre-feeding to reduce food motivation. Pre-feeding for 24 hours suppressed lever pressing on all four schedules tested, including the FR1 schedule. Across all schedules, if data are expressed as a percent of control responding, pre-feeding suppressed responding by approximately 50-60%. Thus, it can hardly be argued that the effects of accumbens DA depletions closely resemble the effects of pre-feeding.
In summary, several lines of evidence indicate that rats with accumbens DA depletions remain directed towards the acquisition and consumption of food, provided that the work requirement is relatively low. Several studies have shown that the effects of accumbens DA depletions do not resemble those of either extinction or pre-feeding. In view of the behavioral research and theory emphasizing that motivation is a critical aspect of primary reinforcement (Bindra, 1978; Thorndike, 1911; Timberlake and Allison, 1974; Timberlake, 1993; see reviews by Salamone, 1992, and Salamone et al., 1997), these results indicate that accumbens DA depletions do not interfere with the primary or unconditioned reinforcing properties of food.
Effects of Accumbens DA Depletions: Importance of Baseline Rate
As described above, the effects of accumbens DA depletions on food-reinforced lever pressing depend markedly upon the nature of the task being assessed. Lever pressing on some schedules (e.g., FR1, VI30) is relatively unaffected by accumbens DA depletions, while other schedules (e.g. FR64) appear to be highly sensitive to the loss of accumbens DA. Studies with the concurrent FR5/feeding procedure indicate that interference with accumbens DA, either by DA depletions or local injections of DA antagonists, affects the relative allocation of instrumental responses with different kinetic requirements.
For several reasons, which were reviewed above, it does not appear as though this pattern of effects is consistent with the DA hypothesis of reward. An alternative hypothesis that we have put forth is that rats with accumbens DA depletions are very sensitive to the kinetic requirements of the instrumental response being performed. In particular, it has been argued that accumbens DA depletions reduce the propensity for expending energy or exerting effort (Cousins and Salamone, 1994; Neill and Justice, 1981, Salamone, 1987, 1988, 1991, 1992; Salamone et al., 1994, 1997; Szechtman et al., 1994; see also Hsiao and Chen, 1995).
An important manifestation of this hypothesis is that instrumental tasks characterized by a high work output should be more greatly affected by accumbens DA depletions than tasks with a low work output. Indeed, it is possible to use this idea to explain the variability in the effects of accumbens DA depletions across different operant schedules. Schedules that generate only 300-600 responses per 30 min (e.g., CRF, fixed and variable interval 30 sec) show little or no effect after accumbens DA depletions (Cousins et al., in press; McCullough et al., 1993a; Salamone et al., 1995; Sokolowski et al., 1998). Schedules that generate moderately high rates (e.g. FR5, FR16, progressive ratio) are substantially impaired by accumbens DA depletions (Aberman et al., in press; Hamill et al., submitted; Salamone et al., 1993b), and schedules generating very high rates (e.g., FR64) are severely impaired (Aberman et al., submitted). In order to illustrate this principle, we have constructed a figure relating the extent of impairment induced by accumbens DA depletions to the baseline rates of responding generated by each schedule (Figure 1).
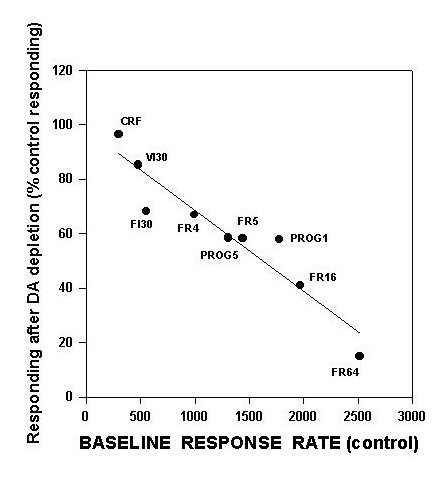 Fig.1: This is a scatterplot, showing the relation between baseline response rate per 30 min (i.e., responding in the vehicle-treated control group) and suppression of responding shown in rats with accumbens DA depletions. Data in this figure are taken from several different studies (i.e., each point represents a separate study; see text). Line represents least-squares regression line, which significantly fit these data. For schedules in which baseline response rate is higher, the suppressive effects of accumbens DA depletions are much greater.
Fig.1: This is a scatterplot, showing the relation between baseline response rate per 30 min (i.e., responding in the vehicle-treated control group) and suppression of responding shown in rats with accumbens DA depletions. Data in this figure are taken from several different studies (i.e., each point represents a separate study; see text). Line represents least-squares regression line, which significantly fit these data. For schedules in which baseline response rate is higher, the suppressive effects of accumbens DA depletions are much greater.
This figure includes data from several separate studies from this laboratory. FR1 data are from Aberman et al. (submitted), and data on FI30 performance are from Cousins et al. (in press), while progressive ratio data are from Hamill et al. (submitted). Aberman et al. (submitted) studied FR4, FR16 and FR64 schedules. As shown in figure 1, there is a linear relation between the baseline rate of responding generated by a schedule and the degree to which that schedule can be suppressed by accumbens DA depletions. Higher baseline rates are associated with greater suppression by accumbens DA depletions, while lower baseline rates are associated with little or no suppressive effects. To emphasize that this precise relation is specific to the effects of accumbens DA depletions, figure 2 also shows an additional line for the effects of pre-feeding (data from Aberman et al., submitted).
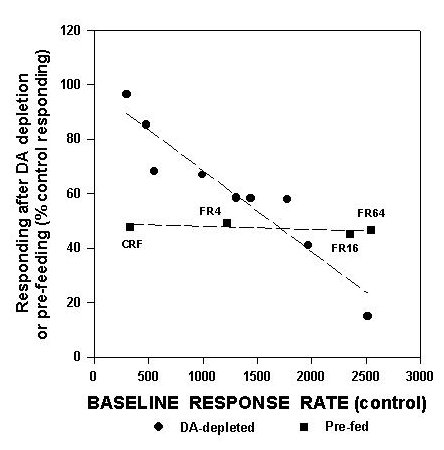 Fig.2: This depicts two scatterplots, one of which shows the relation between baseline response rate per 30 min (i.e., responding in the control condition) and suppression of responding shown in rats that were pre-fed for 24 hrs (squares). Data in this figure are additional analyses performed on data reported in Aberman and Salamone (submitted). Line represents least-squares regression line, which significantly fit these data. Data from figure 1, for DA depleted rats, also are shown (circles). It can be seen that the relation between baseline responding and suppression of responding is substantially different in pre-fed animals than in DA-depleted ones.
Fig.2: This depicts two scatterplots, one of which shows the relation between baseline response rate per 30 min (i.e., responding in the control condition) and suppression of responding shown in rats that were pre-fed for 24 hrs (squares). Data in this figure are additional analyses performed on data reported in Aberman and Salamone (submitted). Line represents least-squares regression line, which significantly fit these data. Data from figure 1, for DA depleted rats, also are shown (circles). It can be seen that the relation between baseline responding and suppression of responding is substantially different in pre-fed animals than in DA-depleted ones.
Although pre-feeding also suppresses responding, it does so in a manner that is substantially different from that of accumbens DA depletions. An examination of these two sets of data clearly demonstrates that accumbens DA depletions produce effects that are quite distinct from those produced by pre-feeding, and also illustrates that the effects of accumbens DA depletions depend highly on the baseline rate generated by the schedule.
The relations depicted in figures 1 and 2 also serve to rebut another point that often is raised by proponents of the anhedonia hypothesis. It is sometimes claimed that the motor effects of interfering with DA systems "mask" the blunting of positive reinforcement that also is produced by the same manipulations. A detailed examination of figures 1-2 would not support that. Accumbens DA depletions do not simply produce severe motor impairments that more greatly affect responding than the "subtle" motivational effects would do. Accumbens DA depletions produce more of a suppressive effect than pre-feeding on high-rate schedules, but actually produce less of an effect than pre-feeding does on low-rate schedules. The FR1 schedule, which is the most fundamental example of a simple, continuous, primary reinforced task, is greatly affected by pre-feeding but is little altered by accumbens DA depletions.
On the Relation Between Accumbens DA Release and Operant Response Rate
The studies reviewed above involve investigations in which the behavioral effects of accumbens DA depletions were assessed. The development of in vivo neurochemical methods has allowed for the possibility of studying behavior and neurotransmitter activity concomitantly (e.g., Salamone et al., 1982, 1989, 1994; see review by Salamone, 1996). Several microdialysis studies have demonstrated that accumbens DA release is positively correlated with lever pressing rate. Responding on either FR1 or avoidance lever pressing schedules increased extracellular DA levels in accumbens, and significant positive linear correlations between the number of responses and the increases in DA were reported (McCullough et al., 1993a, 1993b). During FR5 responding, there also were substantial increases in accumbens extracellular DA over baseline, and a significant hyperbolic relation between responding and increases in DA (Salamone et al., 1994b).
The FI30 schedule is characterized by a higher rate of responding than the FI120 schedule, and also is accompanied by greater increases in DA release (Cousins et al., in press). A recent study (Sokolowski et al., 1998) examined DA release in accumbens core and shell during three operant tasks in the rat, in order to investigate in more detail the relation between DA release and lever pressing rate. Rats were trained to lever press on a fixed-ratio 5, variable-interval 30 sec, or a tandem variable time 30 sec/fixed ratio 5 schedules (referred to as a tandem VI/FR in the article); these three schedules were chosen because they generate a wide range of response and reinforcement rates. Attaching a ratio requirement to the variable time interval generated a very high response rate, but the density of food presentation was approximately the same as the VI 30 sec. After several weeks of training, dialysis probes were implanted into nucleus accumbens core or shell subregions. A single 30 min behavioral test was conducted during the dialysis test session. Rats lever pressing on each of the three operant schedules showed a significant increase in extracellular DA relative to the food-deprived control group during the behavioral session.
In addition, increases in extracellular DA in accumbens shell were found to be significantly greater than in the core during the lever pressing period. Across all three schedules, extracellular DA in the nucleus accumbens was significantly correlated with the number of lever presses performed, but was not correlated with the number of food pellets delivered. Analysis of covariance, which used amount of food consumed as the covariate, showed an overall group difference, indicating that DA levels increased in lever pressing animals even if one corrected for the amount of food consumed. These results indicate that DA release was more responsive in the accumbens shell than in the core during operant responding, and that increases in extracellular DA in nucleus accumbens shown by experienced animals are related to response rate rather than reinforcement magnitude.
Conclusions: Involvement of Accumbens DA in Regulating Energy Output
From an energetic standpoint, individual cells, as well as complex multicellular organisms, are non-equilibrium open systems. Complex organisms must obtain energy by ingesting substances from their environment. Of course, in order to acquire energy in the form of nutrients, animals must expend energy in the form of muscle contractions. The acquisition of food involves more than just consummatory behaviors; it also involves instrumental actions that increase the proximity and availability of food. Because organisms are separated from significant stimuli such as food by environmental constraints or obstacles (i.e., response or procurement "costs"), these instrumental behaviors often are characterized by a high degree of vigor, persistence and work output.
The notion that motivated behaviors have an energetic or activational component is an old one, and there are numerous examples of this idea from the literatures of psychology (e.g., Cofer, 1972; Duffy, 1963; Killeen et al., 1978) and ethology (e.g. Hinde, 1970; Marler and Hamilton, 1966). In addition, this view is consistent with more recent "economic" models of operant conditioning, and the idea that response procurement "costs" affect operant responding (Allison, 1980; Collier and Jennings, 1969; Collier et al., 1986; Gannon et al., 1983; Hursh et al., 1988; Kaufman, 1980; Lea, 1978; Rashotte and Henderson, 1988; Staddon, 1979, 1983). In mammals, the brain is a critical structure for the regulation of complex behavior, and thus it is important to investigate the brain mechanisms involved in activational aspects of instrumental behavior. The major conclusion that can be drawn from the work reviewed above is that release of DA in nucleus accumbens is an important part of the neural process that enables organisms to overcome work-related response costs. In economic terms, nucleus accumbens DA is involved in the elasticity of demand for food.
As described above, studies involving response choice tasks or various schedules of reinforcement have demonstrated that the effects of accumbens DA depletions interact strongly with the work output typically seen on that particular task. Rats with accumbens DA depletions are impaired on operant schedules characterized by high levels of work output, and, on choice procedures, accumbens DA depletions cause animals to shift their relative response allocation in the direction of the task with lower work requirements. In addition, release of DA in nucleus accumbens is correlated with operant response output. Although it is difficult to identify precisely the behavioral functions of nucleus accumbens DA, it does appear that depletions of DA reduce the propensity for expending energy or effort, and that accumbens DA release could be one of the mechanisms through which response output is regulated. The regulation of global aspects of work output can be conceived of as a higher-order sensorimotor process, but also as an aspect of motivation (for reviews, see Salamone, 1987, 1992; Salamone et al., 1997).
It is possible that nucleus accumbens, through its anatomical connections, participates in frontal cortical control of behavioral regulation. Although nucleus accumbens may not directly assess reward value, or perform cost/benefit analyses, it is possible that accumbens DA transmission sets constraints on energy expenditure that profoundly influence the relative allocation of instrumental responses toward various alternatives. As well as shedding light on the neural control of motivated behavior, research into the functions of nucleus accumbens DA also could have important implications for clinical studies of energy-related disorders, such as anergia or apathy (Campbell and Duffy, 1997; Duffy and Kant, 1997).
| Discussion Board | Previous Page | Your Symposium |