Invited Symposium: What Can Genetic Models Tell Us About Attention-Deficit Hyperactivity Disorder (ADHD)?
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Previous research from our laboratory has demonstrated dramatic changes in dopamine receptor density during adolescence (Andersen et al., 1997; Teicher et al., 1995). We and others have hypothesized that the marked overproduction and elimination of synapses and receptors during adolescence may serve as a permissive factor for a number of psychiatric disorders, including schizophrenia (e.g., Weinberger, 1987), attention deficit hyperactivity disorder (ADHD), and Tourette’s Syndrome (Teicher et al, 1997). Understanding the fundamental changes that occur in the brain during the period surrounding adolescence and ascertaining how these changes differ between sexes will bring us closer to understanding a number of important findings about these disorders, especially about ADHD. First, we know that genetic factors play an important role, but are not entirely sufficient, as concordance between monozygotic twins may be as high as 90% in ADHD (reviewed by Teicher et al, 1997). Second, we know that sex differences occur in ADHD, as males are more often diagnosed than females (2-9-fold more prevalent in males; Bird et al 1988; Anderson et al 1987). Third, ADHD has a developmental etiology, as diagnosis must occur by 7 years of age.
Changes in the dopamine system during the periadolescent period are especially important for our understanding of ADHD as pharmacotherapy utilizes dopamimetics, including methylphenidate and amphetamine. Also, the course of ADHD peaks during childhood and wanes with the transition from adolescence to adulthood; in fact 30% of cases remit by adolescence and the number climbs to 50-70% remission by adulthood (Barkley, 1990; Gittelman et al., 1985). For these reasons, we decided to investigate the developmental strategy of overproduction and elimination of dopamine receptors in the striatum and nucleus accumbens. Moreover, we wanted to determine gender differences in this process. Ernst and colleagues (1994) have documented gender differences in overall cerebral glucose levels between boys and girls with ADHD, where ADHD girls have lower glucose metabolism than both normal girls and boys with ADHD.
We now report that male rats exhibit a much greater degree of striatal and accumbens over production and elimination of dopamine D1 and D2 receptors. We also present preliminary data suggesting that the process of receptor overproduction and elimination that occurs during periadolescence is not a consequence of pubertal surges in gonadal hormones.
Materials and Methods
Male and female Sprague-Dawley rats were obtained from Charles River Laboratories at 15 days of age and their brains were removed for autoradiographic analysis at 25, 35, 40, 60, 80, 100, and 120 days of age. Females were sacrificed on the second day of diestrous (determined by a vaginal smear) ±2 days of the same ages. An average of six subjects were studied at each age. Preliminary studies utilized subjects gonadectomized at 28 days of age (n=4). Subjects were treated in accordance with all relevant institutional, state and federal guidelines.
Sections were obtained and treated as previously described (Andersen et al., 1997). Briefly, frozen brains were sectioned coronally in 10 µm slices at -15oC, thaw-mounted on gelatin-coated slides, and stored at -70oC. Slides were thawed to room temperature the day of processing 5 min before incubation in D1 or D2 buffers. Saturation analyses were obtained using seven concentrations of each ligand. D1 receptors were determined using 3H-SCH-23390 (0.05-5 nM; 72.8 Ci/mmol) with 2 µM cis-flupenthixol as the blank. D2 receptor binding was determined with 3H-nemonapride (0.05-3.0 nM; 81.4 Ci/mmol) and 2 µM haloperidol. Specific binding at various concentrations of free ligand were analyzed with LIGAND (Munson and Robard, 1980) to derive estimates of Bmax and Kd, using non-linear regression to a single-site model for each subject. Protein concentrations were determined with the Commassie Blue method in each region across age. Final values for Bmaxwere expressed as fmol/mg protein.
Results
As illustrated in Figure 1, males and females differed markedly in their pattern of dopamine receptor development in striatum (Age x Gender Interaction D1: F6,48 = 11.0, p< 0.0001; D2: F6,42 = 5.60, p< 0.0001). Male D1 and D2 receptor density had a much more prominent rise than in females between 25 days of age and the onset of puberty at 40 days. For example, 2 Bmax increased 144±26% in males vs 31±7% in females. The decline in male receptor density was also more marked between 40 days and adult levels (120 days). D1 Bmax decreased 34±4% in males, but by only 7±8% in females. In short, male rats had more extensive over production and elimination in striatum, though adult values were nearly identical by 120 days. Kd values did not differ between males and females or across age in the striatum.
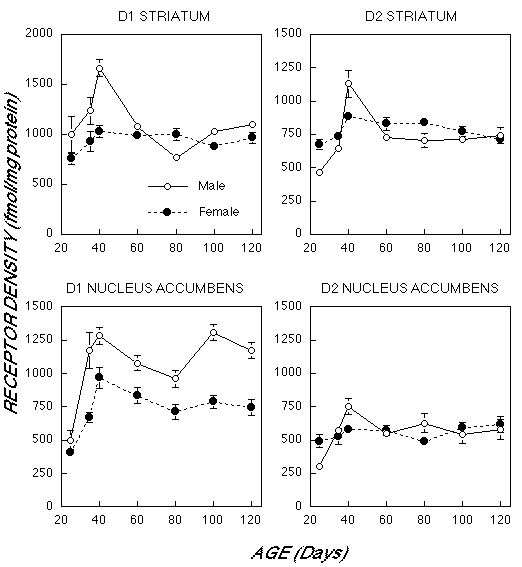 Fig. 1: Mean (±S.E.) Bmax values (expressed as fmol/mg protein) for density of D1 and D2 receptors in male and female rats between 25 and 120 days of age in the striatum and nucleus accumbens based on receptor autoradiography.
Fig. 1: Mean (±S.E.) Bmax values (expressed as fmol/mg protein) for density of D1 and D2 receptors in male and female rats between 25 and 120 days of age in the striatum and nucleus accumbens based on receptor autoradiography.
We also observed differences in the laterality of D1 and D2 receptors in male striatum, but not in female striatum. D1 receptor density was approximately 20-30% higher in the left striatum than the right until 100 days of age, when laterality reversed with right becoming 20% higher. A similar phenomenon was observed for D2 receptors in male striatum, however, receptor asymmetries were no longer observed by 60 days. In contrast, females D1 and D2 receptor density did not differ between right and left hemisphere at all ages.
There were enduring gender differences in D1 receptor density in nucleus accumbens (F1,44 = 109.1, p< 0.0001). Despite comparable densities at 25 days of age, D1 Bmax rose faster and higher in males than females (Age X Gender interaction: F6,44 = 3.31, p< 0.01). The male and female density curves were parallel after 40 days of age, with each demonstrating a slight dip at 80 days. At 120 days D1 Bmax was 57.8± 21.2% greater in males than females.
Overall, there was no gender difference in D2 density in the nucleus accumbens (F1,42 = 0.13, p > 0.7). Males had a greater increase from 25 to 40 days (147±24%) than females (18±9%), but both groups showed no significant variations from 40 to 120 days. Again, no sex or age differences in Kd were observed in nucleus accumbens.
Gonadectomy at 28 days of age, as pubertal hormones are beginning to rise and exert their activational effects, did not significantly effect D1 and D2 receptor density in the striatum of male or female rats (see Table 1). Data are expressed as a percentage of intact control for both castrated (Cx) males and ovariectomized (Ovx) females.
TABLE 1.
----------------------------------------------
Cx Males* Ovx Females*
D1 83.3 ± 15.0 92.9 ± 23.2
D2 76.3 ± 10.0# 86.2 ± 8.5
----------------------------------------------
* data are expressed as a % of control values at P40
# p=0.08
Discussion and Conclusion
The observation of more elaborate over production and elimination of D1 and D2 family receptors in striatum of males than females across periadolescence has important implications for our understanding of ADHD. We hypothesize that the extensive over production of dopamine receptors in striatum and accumbens during prepubertal development may help explain why males are more often afflicted by ADHD as dopaminergic increases in these regions can produce hyperactivity and stereotypies (Niemegeers and Janssen, 1974; Giros et al., 1996). The extensive pruning of dopamine receptors in striatum after puberty is consistent with the observation that ADHD often recedes or diminishes in severity in males. The possibility that either failure of striatal dopamine receptor density to recede in females, or an aberrant overproduction in the females, may explain why ADHD symptoms are more likely to persist after puberty in afflicted females (Ernst et al., 1994). Data from Ernst et al (1994) suggests that ADHD females may actually effected more severely than ADHD males. It is also possible that the enduring differences in D1 density in nucleus accumbens may have some relation to the greater incidence of substance abuse in males given the putative role of the accumbens D1 system in addictive behaviors (Maldonado et al., 1993).
These preclinical data are also consistent with clinical (human autopsy specimens) that demonstrated marked over production and elimination of D1 and D2 receptors in striatal dopamine receptor density during childhood and adolescence (Seeman et al. 1987). Unfortunately, they did not examine gender differences in that study. However, gender differences in periadolescent D1 and D2 receptor changes may be consonant with recent observations with magnetic resonance imaging that shows that the human striatum shrinks during adolescence in boys but not in girls (Giedd et al. 1997). Overall, the present results provide further support for sex differences in brain structure and suggest that the developmental processes of over production and competitive elimination may be involved in the emergence of some of these differences.
The mechanism underlying these sex difference in the overproduction and elimination of dopamine receptor density during periadolescence is not straight forward. The most likely explanation underlying gender differences in dopamine receptors is due to pubertal changes in gonadal hormones, where they take on an activational role. However, as Table 1 illustrates, gonadectomy at postnatal day 28 (as hormone levels are beginning to surge) did not have a dramatic effect on this process. Two hypotheses were discounted: 1) either testosterone stimulates the overproduction in males, which is not the case, as castration did not markedly suppress the receptor density at day 40; or 2) estrogen inhibits the overproduction in females — also not true as no disinhibition of periadolescent receptor overproduction was observed. Thus, the question as to what is the mechanism underlying these gender differences remains to be answered. The answer may lie in the original organizing effects of hormones during early brain development. The current direction of this line of research in our laboratory is to document the effects of chronic methylphenidate on periadolescent changes in dopamine receptor density in males and females.
References
- Andersen SL, Rutstein, M, Benzo JM et al. Sex differences in dopamine receptor overproduction and elimination. NeuroReport 8, 1495-1498 (1997).
- Anderson JC, Williams S, McGee R et al. DSM-III disorders in preadolescent children: prevalence in a large sample from the general population. Arch Gen Psychiatry 44, 69-76 (1987).
- Barkley RA. A critique of current diagnostic criteria for attention deficit hyperactivity disorder: clinical and research implications. J Dev Behav Pediatr 11, 343-52 (1990).
- Bird HR, Canino G, Rubio-Stipec M et al. Estimates of the prevalence of childhood maladjustment in a community survey in Puerto Rico. Arch Gen Psychiatry 45, 1120-1126 (1988).
- Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Arch Gen Psychiatry 53, 607-616 (1996).
- Ernst M, Liebenauer LL, King C, et al. Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry 33, 858-868 (1994).
- Giedd JN, Castellanos FX, Rajapakse JC, et al. Sexual dimorphism of the developing human brain. Prog Neuropsychopharm Bio Psychiatry 21, 1185-1201 (1997).
- Giros M, Jaber M, Jones SR, et al. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606-612 (1996).
- Gittelman R, Mannuzza S, Shenker R et al. Hyperactive boys almost grown up; I: psychiatric status. Arch Gen Psychiatry 42, 937-947 (1985).
- Maldonado R, Robledo P, Chover AJ, et al. Pharmacol Biochem Behav 45, 239-42 (1993).
- Munson P & Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem 107, 220-239 (1980).
- Niemegeers CJE & Janssen PA. Arzneim Forsch 24, 45-52 (1974).
- Seeman P, Bzowej N, Guan H, et al. Human brain receptors in children and aging adults. Synapse 1, 399-404 (1987).
- Teicher MH, Andersen SL, & Hostetter JC. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev Brain Res 89, 167-172 (1995).
- Teicher MH, Andersen, SL, Glod CA, et al Neuropsychiatric disorders of childhood and adolescence. In SC Yudofsky and RE Hales (eds) Textbook of Neuropsychiatry 3rd ed. American Psychiatric Press: Washington DC (1997), pp. 903-941.
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. ArchGen Psychiatry 44, 660-669 (1987).
| Discussion Board | Previous Page | Your Symposium |