Invited
Symposia
Presentation
Have Enzymes of the "Third Pathway" been demonstrated in the Gut?
Do the metabolites produced have biological activity on the intestinal tract?
| INABIS '98 Home Page | Your Symposium | Related Symposia & Posters | Scientific Program | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Oxidation of arachidonic acid (AA) generates a variety of biologically active mediators. Three distinct pathways have been described. The enzyme systems involved are regiospecific and stereospecific. Of the three pathways, considerable attention has been paid to the products of the cyclo-oxygenase and lipoxygenase pathways and their effects on intestinal in transport processes well described. By contrast much less attention has been paid to the products of the so-called "third pathway", the cytochrome P450-dependent mono-oxygenases even though the involvement of this pathway in the metabolism of arachidonic acid was noted in the late sixties (see Capdevila et al.,1992 for historical perspective).
The "third pathway" that is mediated by the CYP enzymes uses NADPH and molecular oxygen in a 1:1 stoichiometry. Three types of oxidative reactions are known to occur ( Rahman et al.,1997, Needleman et al.,1986, Capdevila et al.,1992, and McGiff et al.,1991). Olefin epoxidation (epoxgenases) gives rise to 4 sets of regio-isomers, the epoxyeicosatrienoic acids (EETS). These are the (5,6-), (8,9-), (11,12-) and 14,15-EETs. These in turn can be hydrolysed to form the corresponding dihydrox derivatives (DHETs). Allylic oxidation leads to the formation of hydroxyeicosatetraenoic acids (HETEs). In this group, (5-), (8-) , (9-), (11-), (12-) and 15-HETEs have been identified. a and a-1 oxidation's produce 19- and 20-HETEs of which the latter can be oxidised further to 20-carboxy arachidonic acid (COOH-AA).
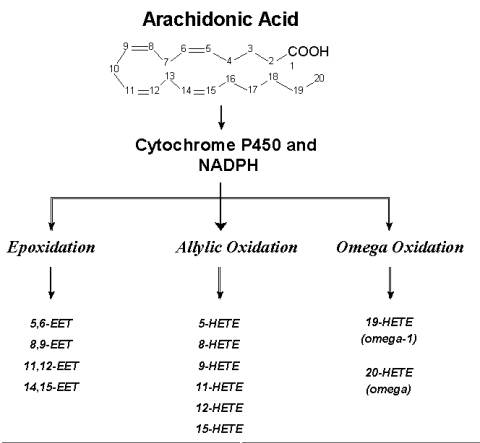
Fig. 1: Arachidonic Acid Metabolism-Oxidative Pathways (Click on a selected pathway to view structural details of the metabolites produced.)
Much of the evidence for the biological effects of the metabolites produced by this pathway comes from studies in the cardiovascular and renal systems, while little documented evidence exists for effects of the products on intestinal ion transport. In a cursory MEDLINE search, we found over 300 publications linking arachidonic acid to P450 enzymes, more than 130 of those dealt with the renal or vascular system. By contrast less than 10 related the two words to either the intestinal or gastrointestinal tract. Even accounting for the errors in searches of this kind, the relatively discrepancies suggest that much work still needs to be done.
In this brief commentary, we will highlight the studies that have been done on the CYP system in the intestine and the potential role of the products in altering intestinal ion transport processes. By pointing to work that has been done in other systems and drawing comparisons, we hope to interest GI investigators in what we believe is an interesting area of research.
To suggest that the "third pathway" is relevant in the gut, it is important to show that; (a) the required enzymes are present in the gut; (b) these enzymes metabolise AA and; (c) the metabolites have demonstrable effects on intestinal ion transport. In the sections to follow an attempt will be made to discuss each of these issues set in the form of specific questions.
Back to the top.(A) Have Enzymes of the "Third Pathway" been demonstrated in the Gut?
A number of P450 enzymes have been demonstrated in the gastrointestinal tract of both humans and experimental animals (Zeldin et al.,1997). These include members of the CYP1A (rats, humans), CYP2B (rats) CYP2C (humans), CYP2D (humans), CYP2E (rats, humans) and CYP3A (rats, humans), CYP2Js (rabbit, rat, humans). Interestingly most of the constitutively expressed CYP proteins showed a proximal to distal gradient, being highest in the duodenum and virtually undetectable in the colon. Again, the expression of most of these appeared to be limited to epithelial cells. The role of most of these enzymes in the metabolism of AA appears to be unclear. More recently attention has been focussed on proteins of the CYP2J subfamily. One particular isoform, CYP2J2 was found to be highly expressed in human cardiac myocytes. Arachidonic acid was metabolised by the enzyme to produce EETs (Wu et al.,1997). CYP2J proteins were also found in ciliated epithelial cells in the airway (Zeldin et al.,1996). A recent study described the expression and localisation of proteins of the CYP2J family in the rat and human gut (Zeldin et al.,1997). In contrast to the other CYP proteins, these were distributed more uniformly along the length of the gut and expression was noted both in epithelial and non-epithelial cells. High levels of the proteins were found in cells of the autonomic ganglia, epithelial cells, intestinal smooth muscle cells and the vascular endothelium. The location of the enzymes suggests that the products of metabolism could have interesting effects.
Since P450 enzymes can catalyse a variety of reactions, it is important to demonstrate that the specific enzymes being considered are capable of metabolising AA. In this context, it has been repeatedly emphasised that stereo specificity of AA product formation must be demonstrated as autooxidation occurs readily and is difficult to eliminate. Stereo selective formation of EETs is regarded as a "decisive criterion" for their enzymatic production (McGiff et al.,1991).
The abilities of several of the enzymes noted above to produce metabolites from AA have been demonstrated in other test systems, principally the liver. Those that can produce EETs include members of the CYP2B, 2C, 1A and 2E subfamilies (Capdevila et al.,1990, Daikh et al.,1994). The particular metabolite produced by each enzyme however was found to vary. Thus in a study of a panel of 10 human enzymes expressed in Hep G2 cells, it was found that CYP 2C8 and 2E formed more of the 14,15- and 11,12- EETs than did CYP 1A2 which appeared to produce more 8,9-EET (Rifkind et al.,1995).
The human jejunum expresses CYP 2J2. In vitro studies showed that the microsomes in the presence of added NADPH converted AA to EETs, their hydration products DHETs, mid-chain HETEs and the C19/20 alcohol's. The EETs and their hydration products accounted for over 50% of the products. In the human jejunum, analysis showed that roughly equal amounts of 14,15- , 11,12- and 8,9 -EETs were present. 5,6 EET was not detected presumably because of extensive degradation (Zeldin et al.,1996).
Studies such as those show clearly that the potential for AA metabolism by P450 enzymes exist in the gut. However the precise products formed and the amounts can only be guessed at. These factors should be taken into account in postulating any physiological or pathophysiological roles for the metabolites.
Back to the top.(C) Do the metabolites produced have biological activity on the intestinal tract?
As noted at the outset, there is scant information on the intestinal effects of AA metabolites through the third pathway. A study in the rabbit intestine suggests that perfusion of the mesenteric vascular bed with ileal microsomal metabolites produced a dose-dependent relaxation of vessels pre-contracted with acetylcholine. Since the extracts contained a variety of HETES, principally 19- and 20-HETES, it could be inferred that these were active vasodilators. Perfusion with authentic HETES produced the same effects (Macica et al.,1993).
However the location of the enzymes as demonstrated by immunohistochemical studies suggest several potential sites. These include the transporting enterocytes, the autonomic ganglia and paracrine or autocrine cells (Zeldin et al.,1997). Inferences can be readily made from published information from other test systems.
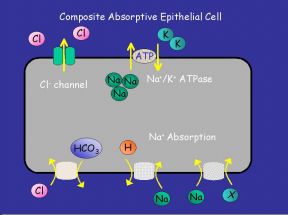 | 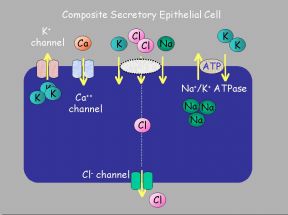 |
Fig. 2: (a) "Generic Absorptive Cell" showing basic ion transport mechanisms involved in absorption. (b) "Generic Secretory Cell" showing basic ion transport mechanisms involved in secretion.
On the transporting enterocytes, EETs can affect a number of transporters directly. These effects can be localised to all or some of the many transporters on the absorptive or secretory cells. Potential sites are shown above on Fig. 2a,b. Evidence from other cell types exist in favour of some of these possibilities.
Effects on K+ channels:
Hyperpolarisation leading to vascular smooth muscle relaxation is linked to stimulation of Ca2+-activated K+ channels (Quilley et al.,1997). 5,6-EET and 11,12- EET can act at this locus (Hu and Kim, 1993). Similar observations have been made on airway smooth muscles as well. In the latter, 5,6- and 11,12-EETs directly activated KCa channels in planar lipid bilayers containing vesicles reconstituted from airway smooth muscles (Dumoulin et al.,1998)
Effects on Ca2+ channels:
Changes in intracellular Ca2+ induced by Angiotensin II in renal proximal tubular cells were enhanced by arachidonic acid and attenuated by an inhibitor of P450, ketoconazole. 5,6-EET but not other regio-isomers (8,9-, 11,12- and 14,15-EET) also augmented the Ca2+ transients, but these effects were not affected by ketoconazole (Madhun et al.,1991).
In rat ventricular myocytes, antimycotics such as clotrimazole, econazole and miconazole, inhibited calcium currents and 11,12-EET enhanced ICa and altered intracellular cAMP concentrations (Xiao et al.,1998). In porcine aortic endothelial cells, CYP 450 metabolites, such as 11,12-EET and 5,6-EET constituted a signal for Ca2+ entry activation (Hoebel et al.,1997) . Ca2+ entry processes into cells are complex. P450 metabolites may be the link between depletion of intracellular stores and Ca2+ entry steps in several cell types (platelets, smooth muscle cells, endothelial cells). The particular metabolite involved may vary. In bovine endothelial cells, 5,6-EET (Graier et al.,1995) was involved whereas in a smooth muscle cell line, 8,9- and 11,12-EETS were effective whereas 5,6- and 14,15-EETS were not (Graber et al.,1997).
Effects on Cl- channels:
Salvail et al.(1998), showed that 5,6- and 11,12-EETs directly inhibited a Ca2+-insensitive Cl- channel from vesicles prepared from bovine tracheal airway smooth smooth muscles and reconstituted into planar bilayer membranes. This could in part explain airway epithelium derived hyperpolarisation of smooth muscle. In rat tracheal epithelial cells, the effects of 11,12-EET were highly stereoselective. It caused a dose-dependent decrease in Isc and Vt, presumably through inhibition of a conductive Cl- pathway. By contrast in the rat colon, 5,6-EET stimulated a furosemide sensitive Isc which was however inhibited by inomethacin. Thus the metabolite in turn produced an eicosanoid (Kockerling et al.,1997).
Effects on Na+ Symports:
In renal epithelial cells, several EETs were tested for their abilities to inhibit Rb2+ uptake. 14,15-EET was the most potent. They argued that this inhibition of Rb2+ uptake was indirect, being linked to the inhibition of a Na+/H+ symport. This evidence was relatively indirect (Staudinger et al.,1994).
In cultured rat glomerular mesangial cells, 14,15-EET was mitogenic and also stimulated Na+/H+ exchange. This was demonstrated by an enhancement of the amiloride-sensitive component of the 22Na uptake following intracellular acidification (Harris et al.,1990)
Effects on Na+/K+ ATPase:
Bovine corneal epithelial microsomes produced several metabolites when incubated in the presence of AA and NADPH-generating system. One of these was found to inhibit the Na+/K+ ATPase. The active compound appeared to be 12(R) HETE. The authour's suggest an endogenous inhibitor of the sodium pump could have functional significance.
Although extrapolation from such studies must be made with caution, the possibilities that these products may modulate variety of transporting elements is real and worth exploring. A possible approach would be to test the effects of certain inhibitors of the CYP enzymes on the transport of interest. If there is a significant inhibitory effect, attempts can be made to by pass the inhibition with AA metabolites. Studies of this kind again need to be done carefully and cautiously. The inhibitors commonly used are not selective and they do have other effects (McGiff, et al.,1991). It may therefore be difficult to pinpoint the particular metabolite involved, however newer, more selective inhibitors are currently being studied (Wang et al.,1998).
Back to the top.- Capdevila, JH et al.(1992) Cytochrome P450 and the arachidonate cascade. FASEB J., 6:731-736.
- Daikh, BE (1994) Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. JPET, 271(3):1427-1433.
- Dumoulin, M et al.(1998) Epoxyeicosatrienoc acids relax airway smooth muscles and directly activate reconstituted KCa channels. Am. J. Physiol., 275(Lung Cell. Mol. Physiol. 19) L423-L431.
- Graber, MN et al.(1997) Recovery of Ca2+ pools and growth in Ca2+ pool-depleted cells is mediated by specific epoxyeicosatrienoic acids derived from arachidonic acid. J. Biol. Chem., 272:29546-29553.
- Graier, WF et al.(1995) Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J. Physiol., 482(2):259-274.
- Harris, RC et al.,(1990) Epoxyeicosatrienoic Acids Activate Na+/H+ Exchange and Are Mitogenic in Cultured Rat Glomerular Mesangial Cells. J. Cellular Physiol.,144:429-437.
- Hoebel, BG et al.(1997) Activation of microsomal cytochrome P450 mono-oxygenase by Ca2+ store depletion and its contribution to Ca2+ entry in porcine aortic endothelial cells. Br. J. Pharm.,121:1579-1588.
- Hu, S et al.(1993) Activation of K+ channel in vascular smooth muscles by cytochrome P450 metabolites of arachidonic acid. Eur. J. Pharmacolocy, 230:215-221.
- Kockerling, A et al.,(1997) Arachidonic acid stimulates fuosemide-sensitive ion transport via cytochorome P-450 metabolites. Abstract--Kongress der Gesellschaft fur Nephrologie mit der Niederlandischen Gesellshaft fur Nephrologie.
- Macica, C et al.(1993) Characterization of cytochrome P-450-dependent arachidonic acid metabolism in rabbit intestine. Am. J. Physiol. 265 (Gastrointest. Liver Physiol. 28):G735-G741.
- Madhun, ZT et al.(1991) An Epoxygenase Metabolite of Arachidonic Acid Mediates Angiotensin II-induced rises in Cytosolic Calcium in Rabbit Proximal Tubule Epithelial Cells. J. Clin. Invest., 88:456-461.
- McGiff, JC et al.(1991) Cytochrome P-450 Metabolism of Arachidonic Acid. Annu. Rev. Pharmacol. Toxicol., 31:339-69.
- Needleman, P et al.(1986) Arachidonic Acid Metabolism. Ann. Rev. Biochem., 55:69-102.
- Pascual, JMS et al.(1998) Epoxygenase Metabolites of Arachidonic Acid Affect Electrophysiologic Properties of Rat Tracheal Epithelial Cells. JPET 286:772-779.
- Quilley, J et al.(1997) Hyperpolarizing factors. Biochem. Pharmacol., 54;10:1059-1070.
- Rahman, M et al.(1997) The Role of the Cytochrome 450-Dependent Metabolites of Arachidonic Acid in Blood Pressure Regulation and Renal Function. Am. J. Hypertens., 10:356-365.
- Rifkind, AB et al.(1995) Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxyengation in human liver microsomes. Arch. Biochem. Biophys., 320(2):380-389.
- Salvail, D et al.(1998) Direct modulation of tracheal Cl--channel activity by 5,6- and 11,12-EET. Am. J. Physiol., 275(Lung Cell. Mol. Physiol. 19) L432-L441.
- Schwartzman, ML et al.,(1987) 12(R)-Hydroxyicosatetraenoic acid: A cytochrome P450-dependent arachidionate metabolite that inhibits Na+,K+-ATPase in the cornea. Proc. Natl. Acad. Sci. USA, 84:8125-8129.
- Staudinger, R et al.(1994) Effects of Epoxyeicosatrienoic acids on 86Rb Uptake in Renal Epithelial Cells. J. Cell. Physiol., 160:69-74.
- Wang, MH et al.(1998) Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. JPET, 284(3):966-973.
- Wu, S et al.(1997) Molecular Cloning, Expression, and Functional Significance of a Cytochrome P450 Highly Expressed in Rat Heart Myocytes. J. Biol. Chem., 272:12551-12559.
- Xiao, Y-F et al.(1998) Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J. Physiol., 508(3):777-792.
- Zeldin, DC et al.(1996) CYP2J Subfamily Cytochrome P450s in the Lung: Expression, Localizaiton, and Potential Functional Significance. Molecular Pharmacology, 50:1111-1117.
- Zeldin, DC et al.(1997) CYP2J Subfamily Cytochrome P450s in the Gastrointestinal Tract: Expression, Localizaiton, and Potential Functional Significance. Molecular Pharmacology, 51:931-943
| Discussion Board | Previous Page | Your Symposium |