Invited Symposium: Photodynamic Therapy
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
SUMMARY
While PDT is obtaining regulatory approvals for treatment of a wide range of malignant and some non-malignant conditions, research continues to elucidate the mechanisms responsible for its clinical effectiveness. PDT requires the activation of a photosensitizing agent, in the presence of oxygen, by visible light of the appropriate wavelength to generate cytotoxic oxygen species, especially singlet oxygen (1O2). The cellular and tissue targets of PDT are numerous, resulting in a very complex interaction of many diverse effects. It is being realized that at least three components are necessary to achieve long-term tumor control - 1) tumor cell ablation by direct photodynamic damage, 2) damage to the tumor and surrounding normal microvasculature, 3) participation of the host inflammatory and immune system. Preclinical studies show tumor cytoreduction through direct PDT effects to be less than 1-2 logs, far short of the 6-8 logs required for tumor cure.
Limitations to direct tumor cell photodamage appear to include inhomogenious photosensitizer tissue distribution and shortage of ground state oxygen during tumor illumination. It is well established that in PDT of solid tumors the fluence rate of therapeutic light delivery can significantly influence treatment outcome. This can be attributed to the fluence rate dependence of tissue oxygenation. High tissue oxygen consumption during tumor illumination.
It is well established that in PDT of solid tumors the fluence rate of therapeutic light delivery can significantly influence treatment outcome. This can be attributed to the fluence rate dependence of tissue oxygenation. High tissue oxygen consumption during delivery of light at high fluence rate has been demonstrated, with a resulting oxygen deficit for singlet oxygen generation and therefore less efficient treatment. Conversely, maintenance of tumor oxygenation during PDT with light at low fluence rate can also be demonstrated , with a resulting increase in treatment efficiency. Increased PDT efficiency is, at least in part, due to enhanced direct tumor cell photodamage, as well as to effects of fluence rate on the vascular response, especially that of the tumor surrounding normal vasculature.
Preclinically, PhotofrinÒ PDT at high fluence rate (150 mW/cm2) can almost completely protect the normal skin vasculature, while low fluence rate treatment (30 mW/cm2) can lead to total vascular collapse within 1 hour. Collapse of the tumor vasculature occurs regardless of fluence rate. Administration of the nitric oxide synthase inhibitor L-NNA can abrogate the protection of the normal vasculature by high fluence rate treatment and enhance long term tumor control, but has no effect with low fluence rate PDT. Fluence rate dependent changes in tumor oxygenation during PDT have also been observed in patients. Tumor pO2 histograms of clinical measurements in basal cell carcinoma lesions during Photofrin® (1 mg/kg) PDT show a marked shift towards hypoxia with an incident light fluence rate of 150 mW/cm2 and an apparent shift towards increased oxygenation with an incident fluence rate of 30 mW/cm2.
It is too early to determine whether this increased oxygenation during treatment will translate into therapeutic gain.Photodynamic Therapy (PDT) passed a major hurdle towards general acceptence in the medical community when it obtained governmental approval in the United States, first in 1995 for treatment of advanced esophageal cancer and then in early 1998 for treatment of early lung cancer. Approvals in numerous other countries, including Canada, Japan, Germany, France and The Netherlands for a wide range of medical indications preceeded the US approvals. These approvals are restricted to the use of the photosensitizing agent PhotofrinÒ, the first such agent to reach the clinic. Intensive efforts are underway to further expand the range of approved clinical applications as well as to improve the treatment modality by introducing novel photosensitizers and optimizing PDT delivery. To do this successfully we must obtain a complete understanding of the molecular, cellular and tissue PDT response mechanisms. PDT requires the activation of a photosensitizing agent by visible light of the appropriate wavelength to generate cytotoxic oxygen species, especially singlet oxygen (1O2). The source of the photosensitizer can be exogenous (i. e. systemic administration of certain derivatives of phorphyrins, chlorins, phthalocyanines etc.) or endogenous upon administration of a pro-drug such as d-aminolevulinic acid (ALA) which is converted by cells to the photosensitizing agent protoporphyrin IX (PpIX). As the diffusion distance of 1O2 in biological systems is < 0.02 mm {7403}, photodamage is restricted to the site of photosensitizer binding in the cell/tissue.
The cellular targets of PDT are photosensitizer dependent and numerous. Cellular membrane systems are especially vulnerable to photooxidation and therefore the plasma membrane, nuclear membrane, mitochondria and lysosomes may be affected {7404,7405}.
PDT derives its selectivity for destroying tumors from a somewhat preferential accumulation of the sensitizer in malignant tissue as well as from the light delivery which is targeted to the tumor. However, the tissue targets of PDT are also numerous and the tissue responses are also photosensitizer dependent. It is being realized that at least three components are necessary to achieve long-term tumor control - 1) tumor cell ablation by direct photodamage, 2) damage to the tumor and surrounding normal microvasculature, and 3) participation of the host inflammatory and immune system. PDT effects on all these systems appear to influence each other, producing a plethora of responses; however, the relative importance of each for the overall tumor response has yet to be fully defined.
Materials and Methods
PHOTO-DESTRUCTION OF TUMOR CELLS IN VIVO
While it is certain, at least in preclinical model systems, that in vivo exposure of tumors to PDT can rapidly (over 24 - 48 hours) and drastically (at least 3 - 4 logs) reduce the number of clonogenic tumor cells, only a relatively small fraction of this reduction occurs through direct photodamage. Preclinical studies employing very effective and/or curative treatments with a number of photosensitizers, including PhotofrinÒ, bacteriochlorophyll-a, several aluminum phthalocyanine derivatives, a benzophenothiazine and ALA/PpIX showed direct photodynamic tumor cell kill in all cases to be less than two logs and in most cases less than 1 log, i.e. far short of the 6-8 log reduction required for tumor cure {66,5318,5321,6924,6878,7281,7265}.
While in vitro light exposure of tumor cells isolated from in vivo photosensitized tumors predicts that curative reductions of clonogenic tumor cells through direct photodamage might be possible with some photosensitizers {66,5321,5737}, limitations appear to exist which do not allow these reductions to be realized for in vivo PDT treatment. Inhomogenious photosensitizer distribution within the tumor might be one of these limitations. Apart from gross tumor heterogeneity, Korbelik and Krosl {6900} have shown that both photosensitizer accumulation and direct tumor cell kill decrease with the distance of tumor cells from the vascular supply. Another possible obstacle to more effective direct tumor cell photodamage might be limitation of tissue oxygen required for 1O2 generation, as discussed in more detail below.
If direct photodamage only accounts for 1 - 2 logs of tumor cell kill, what can be responsible for the additional orders of magnitude of cytoreduction observed after PDT and leading to tumor cure? It appears that this cell death is secondary to effects exerted upon the tumor bed which come into play after PDT delivery is completed. These include delayed vascular occlusion with the associated nutritional and oxygen deprivation of tumor cells, and effects mediated by the inflammatory and immune responses to PDT.
TISSUE OXYGENATION AND PHOTODYNAMIC OXYGEN DEPLETION
Since 1O2 is generated by energy transfer from the excited photosensitizer to ground state oxygen, it follows that any restriction of tissue oxygen supply during PDT light delivery will have negative consequences for treatment outcome. Such restriction can arise from photochemical oxygen consumption or acute vasoconstriction.
Very rapid reductions in tissue oxygen tensions upon illumination of photosensitized tissue were detected by Tromberg et al. {220}, an observation recently confirmed by others {7266,7265}. Mathematical modeling by Foster et al. {115} demonstrates that the rate of oxygen consumption during Photofrin® PDT can be significant enough to move a fraction of the tumor into very low levels of oxygenation, outpacing the rate of oxygen diffusion from the capillaries, and shrinking the radius of oxygenated tissue volume around them. The rates of 1O2 generation and therefore tissue oxygen consumption are high when tissue photosensitizer levels and the light dose rate of treatment are high {6951,7265,7266}. The light dose rate can be adjusted downward to slow oxygen consumption sufficiently to facilitate the maintenance of (tumor) tissue pO2 levels during treatment {7265}. An important parameter influencing the rate of tissue oxygen consumption is photobleaching of the sensitizer because the reduction of sensitizer levels also reduces the rate of photochemical oxygen consumption {7021}. Another approach towards maintenance of tissue oxygenation during PDT is the hyperfractionation of light delivery, first suggested by Gibson et al. {213} and Foster et al. {115}, consisting of very short (in the order of 20-50 seconds) light and dark intervals, allowing reoxygenation during the dark periods {7266}.
Preclinical evidence indicates that the maintenance of high tumor pO2 levels during PDT is beneficial for the direct photodynamic ablation of tumor cells, although the gain may be less than one additional log of tumor cell kill {6951,6285,7164}. Generally, treatment regimes that are designed to maintain tumor pO2 (low light dose rate, intermittent light) show superior effectiveness in delaying tumor regrowth {213,115,7265,7097,6880,6962}.
We have also observed tissue oxygen depletion in basal cell carcinoma lesions in patients undergoing PhotofrinÒ (1 mg/kg) PDT. Preliminary data are shown in Table 1. Light was delivered at fluence rates of 150 mW/cm2 or 30 mW/cm2 and tissue oxygen pressure was measured before and during light exposure with the polarographic needle probe of the Eppendorf pO2 Histograph.
Table 1. Changes in oxygen pressure in basal cell carcinoma lesions during PDT (1 mg/kg Photofrin®) light treatment.
| Treatment Fluence Rate | Measurement Time | # of Values | Mean pO2 mmHg |
Median pO2 mmHg |
% of Values <2.5 mm Hg |
|---|---|---|---|---|---|
| Pre-light 150 mW/cm2 | n. a. 0.5-2 min 2-10 min |
124 83 94 |
28.9 13.3 19.6 |
29.9 3.9 6.7 |
4.0 47.0 39.4 |
| Pre-light 30 mW/cm2 | n. a. 3-6 min |
104 208 |
24.9 40.7 |
19.1 41.1 |
0.0 1.4 |
Exposure to light at 150 mW/cm2 caused marked acute as well as lasting reductions in mean and median pO2 and corresponding large increases in pO2 values below 2.5 mm Hg, all indicating a shift to severe tumor hypoxia during light treatment. In contrast, exposure to light at 30 mW/cm2 did not show such a shift but rather tended to increase tumor oxygenation, as indicated by an increase in mean and median pO2 values. The observations are consistent with fluence rate dependent photodynamic oxygen depletion in photosensitized tissue, but changes in tumor perfusion during treatment cannot be ruled out as cause for these oxygenation shifts. Follow-up will have to show whether the oxygenation status of a given lesion during treatment has any influence of treatment outcome.
VASCULAR PDT EFFECTS
In addition to photochemical oxygen depletion, oxygen supply in the tissue can be diminished by the damaging effects of PDT on the microvasculature. With certain photosensitizers such as PhotofrinÒ and high photosensitizer doses, blood flow obstruction can be marked and rapid enough to limit the oxygen supply to the tumor during PDT light delivery {630}. With certain second generation sensitizers, many of which exert less severe acute effects on the vasculature than PhotofrinÒ, and lower drug doses, such acute vascular effects are less likely to occur.
PDT induced vascular damage is a two-edged sword. While it can be detrimental to the PDT response when occurring during treatment, it appears to be beneficial when occurring after completion of the PDT tumor treatment, and to contribute significantly to long-term tumor control. Delayed microvascular collapse can be readily observed in clinical lesions following PDT, and has been demonstrated preclinically with many photosensitizers {1772,6060,7161,7160,6924}. It can lead to severe and persistent post-PDT tumor hypoxia/anoxia {2055,6995}. The mechanisms underlying the vascular effects of PDT differ greatly with different photosensitizers. PhotofrinÒ-PDT leads to vessel constriction, macromolecular vessel leakage, leukocyte adhesion and thrombus formation, all apparently linked to platelet activation and release of thromboxane {6876,7291}. PDT with certain phthalocyanine derivatives causes primarily vascular leakage {6877}, and PDT with mono-L-aspartyl chlorin e6 (NPe6) results in blood flow stasis primarily because of platelet aggregation {7288}. All these effects may include components related to damage of the vascular endothelium. PDT may also lead to vessel constriction via inhibition of the production or release of nitric oxide (NO) by the endothelium {5902}. The causality and interconnectedness of these events are still under investigation. In preclinical experiments, the microvascular PDT responses can be partially or completely inhibited by the administration of agents which affect eicosanoid generation such as indomethacin {247}, various other thromboxane inhibitors {6876} and aspirin {6506,6403}, and this inhibition can markedly diminish the tumor response. On the other hand, administration of agents inhibiting nitric oxide synthase (NOS) or scavenging nitric oxide (NO) appears to enhance tumor cure, apparently by enhancing the PDT-induced disruption of vascular perfusion ({7292}, own unpublished observation).
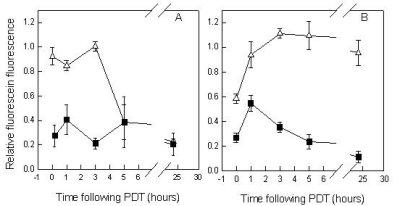 Figure 1. Effects of L-NNA on murine skin perfusion following Photofrin® PDT at 30 mW/cm2 (A) or 150 mW/cm2 (B). (r) without L-NNA, (¾) with L-NNA; L-NNA was injected 5 min prior to low fluence rate illumination and 1 h prior to high fluence rate illumination. Perfusion was determined using the fluorescein dye exclusion assay; fluorescein was injected following PDT at the times indicated and fluorescence was measured within 5 min with a specially designed probe; measurements were taken from the PDT exposed and unexposed skin; data represent the fluorescein fluorescence ratios of treated/untreated skin. Each point is the mean ± SE from at least three animals.
Figure 1. Effects of L-NNA on murine skin perfusion following Photofrin® PDT at 30 mW/cm2 (A) or 150 mW/cm2 (B). (r) without L-NNA, (¾) with L-NNA; L-NNA was injected 5 min prior to low fluence rate illumination and 1 h prior to high fluence rate illumination. Perfusion was determined using the fluorescein dye exclusion assay; fluorescein was injected following PDT at the times indicated and fluorescence was measured within 5 min with a specially designed probe; measurements were taken from the PDT exposed and unexposed skin; data represent the fluorescein fluorescence ratios of treated/untreated skin. Each point is the mean ± SE from at least three animals.
While light fluence rate plays a major role in the tumor oxygenation status during PDT exposure, it appears to have little effect on tumor oxygenation following treatment. With most photosensitizers both oxygenation and perfusion of tumors decrease severely, regardless of fluence rate, with time after treatment due to vascular damage {7265,7160,7161}.
This is in contrast to the response of the normal microvasculature. Recent preclinical studies have shown that, at least with PhotofrinÒ-PDT, low fluence rate treatment can lead to shut-down of normal microvascular perfusion following PDT, while with high fluence rate treatment microvascular patency is maintained. The reasons for this behavior are unclear. Interestingly, this "protection" of the microvasculature by high fluence rate PDT could be abolished by administration of the NO synthase inhibitor NG-nitro-L-arginine (L-NNA) (Figure 1). This implies the involvement of nitric oxide, the absence of which may enhance adhesion of neutrophils at the endothelial surface and thus contribute to vascular occlusion {7404}. Interestingly, high fluence rate PDT treatment by itself inhibited tumor curability, implying that the protection of the tumor-surrounding normal vasculature adversely affected long-term tumor control. The administration of L-NNA prior to PDT that abolished this protection enhanced tumor curability with high fluence rate treatment, but had no effect at low fluence rate. These observations seem to differ from earlier observations that suggested that normal and tumor vessels responded similarly to PDT, both qualitatively and quantitatively {5888}.
Discussion and Conclusion
In conclusion, studies over the past decade have revealed a complex, multi-faceted picture of the tissue/tumor response to PDT, and have highlighted the central role played by oxygen. They have demonstrated how PDT induced changes in tissue/tumor oxygenation can be both beneficial and detrimental to this response. Now further technological development is needed to create ways to monitor in real time oxygen and/or oxygen derived species in the clinical setting to guide us to the most effective and efficient PDT delivery.
| Discussion Board | Previous Page | Your Symposium |