Invited Symposium: Hypertension III: Flow-Induced Vascular Remodeling
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Arterial occlusion leads to increased blood flow through alternate or collateral channels to maintain tissue perfusion. On an acute basis, such increases in arterial blood flow induce vasodilatation in muscular arteries and resistance arteries/arterioles, including arcading arterioles that are candidate collateral channels in ischemia [1,2]. When blood flow is chronically altered, changes in vasomotor tone can be accompanied (or replaced) by changes in arterial structure and function [1,3-5]. Structural alterations seem to normalize perturbations in fluid shear stress along the vascular wall [3,6].
Nitric oxide (NO) is clearly involved in flow-induced vasodilator responses and helps maintain dilatation of collaterals on a chronic basis [7,8]. However, in several pathologies such as diabetes, hyperlipidemia and hypertension, nitric oxide levels may be reduced [9,10]. This study addresses the role of nitric oxide in arterial adaptations to chronic changes in blood flow.
Accordingly, we induced chronic changes in blood flow by ligating every other first order side branch of the superior mesenteric artery in a section of the mesentery, thereby reducing flow in the ligated vessels and increasing flow in the intermediate vessels. We measured blood flow in these first order vessels immediately after surgery and then again two weeks later. To discern the role of nitric oxide, these measurements were made in untreated rats and in rats which received 25 mg/kg/day L-NAME s.c. beginning on the day of surgery. Furthermore, we measured the in vivo diameters of arcading arterioles running along the mesenteric wall and serving as a collateral pathway perfusing tissues distal to the ligation. Diameter measurements were made in ligated and non-operated sections of the mesentery in both untreated and L-NAME treated rats.
Materials and Methods
Surgery and treatment In 8 week old male Wistar Kyoto rats under perntobarbital anesthesia (60 mg/kg i.p.), sections of the intestine were exteriorized via a midline incision and held moist with saline-wetted gauze. Beginning from either the stomach or the coecum, every other first order mesenteric side branch was ligated (5-0 silk) until a total of four ligated vessels was reached. This procedure resulted in reducing blood flow in ligated vessels (LO) and increasing blood flow in the intermediate parallel vessels (HI). After the ligations had been made, the intestines were returned to the abdomen and the wound was sutured with 3-0 silk. This model is an adaptation of our earlier ligation model [1] since vessels from the distant unoperated part of the vascular bed could be used as internal controls (CON). Immediately after surgery, rats received either no treatment (NT) or chornic administration of L-nitro-arginine methylester (L-NAME; 25mg/kg/day s.c. for 2 weeks; Sigma, St. Louis MO, USA) via osmotic mini-pumps (2002, Alzet, Alza Co., Palo Alto CA, USA). After surgery the rats were allowed to recover and had free access to chow (Hope Farms, Woerden, the Metherlands) and tap water. While under anesthesia, rats were kept warm with a thermostatically- controlled heating pad. All procedures were approved by the ethical committee for animal welfare of the Universiteit Maastricht. Blood flow measurements After the ligations had been made (t0), a micromanipulator was used to position a transit-time ultrasonic flow probe (0.5 mm V-series, Transonic Systems, Ithaca NY, USA) around HI, LO, and CON arteries. Blood flow was measured with a T106 flow meter (Transonic Systems) linked to an on-line computer monitoring system (Hemodynamic Data Aquisition System, Technical Services, Universiteit Maastricht). Flow was sampled at 100 Hz; the values were averaged and recorded every second. Mean flow was measured for a period of at least five minutes after the signal had stabilized in n= 13 rats. Two weeks after the arterial ligations (t2), blood flow was measured in HI, LO and CON arteries in both NT(n=12) and L-NAME (n=9) treated rats under pentobarbital anesthesia. Likewise, blood flow was measured in mesenteric arteries of unoperated 10 week old male rats (n=6; sham). Blood pressure measurements Twelve days after arterial ligation surgery, NT (n=8) and L-NAME treated (n=8) rats were anesthetized with pentobarbital and equipped with a saline-filled catheter advanced from the femoral artery into the abdominal aorta (PE10 heat-sealed to PE50). The catheter was exteriorized at the neck and closed with a metal plug. On the morning of the fourteenth day after surgery, mean blood pressure was monitored via a pressure transducer (CP-01, Century Technology CO., Inglewood Ca, USA) in conjunction with the above-mentioned computer program. Arteriolar diameter measurements In other groups of L-NAME treated (n=7) and NT (n=7) rats with mesenteric artery ligations, the diameters of arcading arterioles running along the intestinal wall between two CON arteries (conarc) or between a HI and LO (ligarc) artery were monitored. To do so, rats were anesthetized (pentobarbital) and a mesenteric loop was exteriorized via a lateral incision. The exteriorized mesentery was held moist by the superfusion of warmed Krebs buffer. A shearing monitor was used to determine arteriolar diameter after the apporpriate arteriole had been freed from surrounding fat tissue. Arteriolar diameters were recorded under basal conditions and during the superfusion of 0.1 mM sodium nitroprusside (SNP) in warmed Krebs buffer. In previous pilot experiments, SNP was observed to induce maximal dilatation of mesenteric muscular arteries. On average, two arterioles from both categories were measured and averaged per rat. Statistics Results are presented as means S.E.M. Differences within a treatment group were compared by a Wilcoxon signed ranks test. Differences between two treatment groups were compared by a Mann Whitney rank test. P values of < 0.05 were considered to be statistically significant.
Results
Neither arterial ligation, nor L-NAME treatment had an effect on body weights (sham:253±8g; untreated: 252±4g; treated:250±2g). Mean blood pressure was elevated during chronic L-NAME treatment (148±4 vs. 119±5 mmHg). No differences in flow were observed between CON (0.26±0.05 ml/min) or sham (0.21±0.03 ml/min). Ligation of NT arteries reduced flow at t0 (0.03±0.01 ml/min) in LO, which remained low at t2 (0.04±0.01 ml/min). HI arteries of NT rats had significantly increased flows at t0 (0.45±0.05 ml/min) which remained incresed at t2 (0.55±0.07 ml/min). As shown in Fig.1, chronic L-NAME treatment did not result in significantly altered blood flow in CON (0.18±0.05 ml/min) or LO (0.04±0.01 ml/min), but prevented the blood flow increase in HI (0.29±0.05 ml/min).
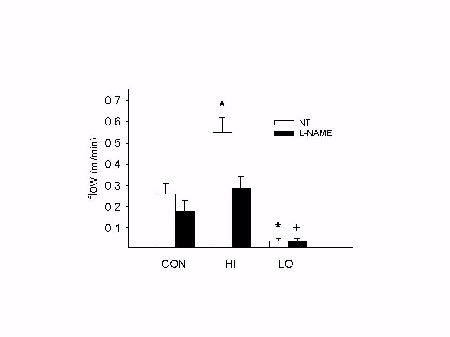 Fig. 1: Chronic L-NAME treatment prevents increase in blood flow in HI vessels. See text for details. * p < 0.05 vs. CON. + p < 0.05 vs. NT.
Fig. 1: Chronic L-NAME treatment prevents increase in blood flow in HI vessels. See text for details. * p < 0.05 vs. CON. + p < 0.05 vs. NT.
Both conarc and ligarc vessels from NT and L-NAME treated rats dilated with 0.1 mM SNP (Fig 2). Under basal conditions, ligarc arterioles were significanlty larger than conarc arterioles in NT rats (249±14 vs. 191±14 micrometer) but not in L-NAME treated rats (191±11 vs. 176±24) . Ligarc arterioles were smaller in L-NAME treated rats than NT rats at basal tone in vivo (191±11 vs. 249±14) and after dilatation with 0.1 mM SNP (225±13 vs. 294±22).
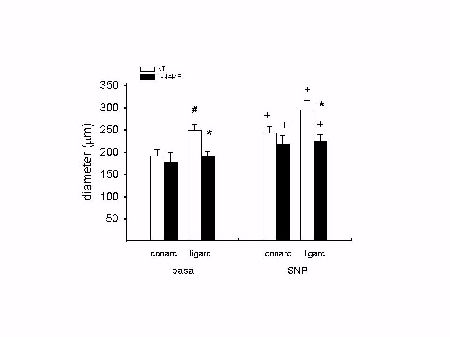 Fig. 2: Arcading arterioles are smaller in L-NAME treated rats. + p < 0.05 vs. corresponding basal measurement; * p < 0.05 vs. corresponding NT group; # p < 0.05 vs. conarc vessels.
Fig. 2: Arcading arterioles are smaller in L-NAME treated rats. + p < 0.05 vs. corresponding basal measurement; * p < 0.05 vs. corresponding NT group; # p < 0.05 vs. conarc vessels.
Discussion and Conclusion
The present model of small mesenteric artery ligation leads to chronically reduced blood flow in ligated arteries (LO) and to chronically increased blood flow in intermediate parallel arteries (HI), but does not disturb blood flow in arteries on the opposite side of the mesentery (CON). Moreover, altering the blood flow through first order mesenteric arteries leads to increased diameters in the connecting arcading arterioles (ligarc) compared to those arcading arterioles connecting control arteries (conarc). Furthermore, our results demonstrate that chronic treatment with the NO synthase inhibitor L-NAME does not effect blood flow through control arteries but prevents the increase in flow in the intermediate parallel arteries (HI). Correspondingly, the ligarc arterioles from L-NAME treated rats were smaller than those from nontreated rats.
The arteries and arterioles of collateral pathways dilate to post-occlusion increases in blood flow. This dilatation is at least in part mediated by nitric oxide on both an acute and a chronic basis [7,8]. Although it has been demonstrated that chronically altered blood flow induces changes in arterial structure and function [1,3-5], the role of nitric oxide in these chronic adaptations has not been addressed. Since reduced nitric oxide levels characterize several pathologies such as diabetes, hyperlipidemia and hypertension [9,10], this role may be clinically relevant.
The present study demonstrates that the diameters of the arcading arterioles connecting arteries with altered flow (ligarc) are significantly smaller in L-NAME treated rats than in nontreated rats under basal conditions in vivo and after maximal vasodilatation with SNP. These results suggest that when nitrc oxide levels are chronically reduced with L-NAME, the flow-induced remodelling in these arcading arterioles (ligarc) is perturbed. The pertinent question which remains concerns the sequence of events preceding the perturbed adaptation. As flow was not increased in the first order arteries, it is possible that L-NAME prevented flow-induced dilatation in these arteries and that no other vasodilator compensated for the decrease in nitric oxide. Ultimately, the lack of dilatation proximally prevented the distal flow increase thereby eliminating the stimulus for dilatation and subsequent remodelling. On the other hand, it is likewise possible that L-NAME directly prevented the dilatation/remodelling in the arcading arterioles which in turn prevented the increase in flow through the first order arteries.
Regardless of the mechanism, although NO synthase inhibition did not compromise perfusion of the intact mesenteric circulation, it persistently prevented the fall in resistance offerred by arcading collateral arterioles. We speculate that the "endothelium dysfunction" observed in hypertension, diabetes, and hyperlipidemia may impede the formation of adequate collateral circulations.
References
- Pourageaud F. De Mey JGR. Structural properties of rat mesenteric small arteries after four week exposure to elevated or reduced blood flow. Am J Physiol 1997:273:H1699- H1706.
- Brownlee RD. Langille BL. Arterial adaptations to altered blood flow. Can J Physiol Pharmacol 1991:69:978-983.
- Driss AB. Benessiano J. Poitevin P. Levy BI. Michel J-B. Arterial expansive remodeling induced by high flow rates. Am J Physiol 1997:272:H851-H858.
- Unthank JL. Fath SW. Burkhart HM. Miller SC. Dalsing MC. Wall remodeling during luminal expansion of mesenteric arterial collaterals in the rat. Circ Res 1996:79:1015-1023.
- Anaizi NH. Swenson C. instability of aqueous captopril solutions. Am J Hosp Pharm 1993:50:486-488.
- Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol 1996:74:834-841.
- Unthank JL. Nixon JC. Dalsing MC. Nirtic oxide maintains dilation of immature and mature collaterals in rat hindlimb. J Vasc Res 1996:33:471-479.
- Morbidelli L. Chang CH. Douglas JG. Granger HJ. Ledda F. Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol 1996:270:H411- H415.
- Vanhoutte PM. Endothelial dysfunction and atherosclerosis. Eur Heart J 1997:18:E19-E29.
- Lüscher TF. Boulanger CM. Yang Z. Noll G. Dohi Y. Interactions between endothelium- derived relaxing factors in health and cardiovascular disease. Circulation 1993:87:V36- V44.
| Discussion Board | Previous Page | Your Symposium |