Pharmacology & Toxicology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Phenobarbital has poor aqueous solubility. Cosolvents are therefore necessary to formulate an aqueous solution. In the US, oral preparations include tablets and an elixir which contains phenobarbital in an alcohol and sucrose base. For neonates there is need for an easy to administer liquid oral dosage form of phenobarbital without cosolvents like alcohol. A suspension may be considered, but has some practical disadvantages.
Recently much attention has been given to the use of lipid microemulsions and monoglycerides in drug delivery [1,2]. To formulate a liquid oral delivery system for phenobarbital we used Myvacet 9-08 as a solvent, without addition of any other excipient. Myvacet 9-08 is an acetic ester of monoglycerides made from hydrogenated coconut oil. The acceptable daily intake for man (ADI) is unlimited. [3,4] We performed an open randomized single dose cross-over study in healthy volunteers to determine the relative bioavailabilty of a solution of phenobarbital in Myvacet 9-08, and a suspension, in comparison with a tablet.
Materials and Methods
Drug Formulation
A:Phenobarbital 100 mg = 5 ml as solution in Myvacet 9-08;
B: Phenobarbital 100 mg = 25 ml as suspension, extemporaneously prepared with an aqueous suspension vehicle
C: Phenobarbital 100 mg tablet
.
Volunteers
The study protocol and written informed consent were approved by the local Institutional Review Board. Seven healthy male volunteers (mean age: 32 yr; mean weight: 75 kg) gave their written consent to participate.
Study-design
On three occasions separated by a 4-week time interval each subject received one of the preparations of phenobarbital. At 8 o'clock in the morning after an overnight fast the drugs were administered to the volunteers with 200 ml of water. No beverages and food were allowed for two hours postdose. All subjects stayed in the hospital for at least 8 h after administration of the phenobarbital. Blood samples were taken 0, 0,25, 0,5 0,75, 1, 2, 3, 4, 5, 6, 8, 24 and 48 hr after intake of the medication.
Analysis of Phenobarbital
A sensitive and validated HPLC method was used for the analysis of phenobarbital.
Pharmacokinetic Parameters
From the individual serum concentration-versus-time curves Cmax and Tmax were determined. For each curve AUC0-48 was calculated by the linear trapezoidal method.
Statistical Analysis
After logarithmical transformation the values for Cmax and AUC0-48 were subjected to an analysis of variance (ANOVA). Values for Tmax were also statistically analyzed.
Results
Other than some mild transient drowsiness no adverse reactions were reported. Most volunteers disliked the taste of phenobarbital in Myvacet 9-08.
Figure 1 shows the serum concentration-versus time profiles of one subject during the initial 10 h after administration of the medication.
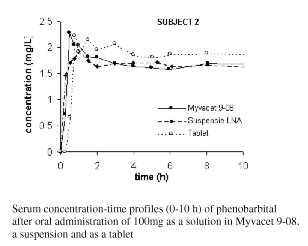 Figure 1
Figure 1
Table 1 summarizes the mean pharmacokinetic parameters of phenobarbital after administration of the three preparations.
 Table 1
Table 1
Table 2 shows the statistical analysis on Cmax and AUC0-48. All three oral dosage forms of phenobarbital are bioequivalent.
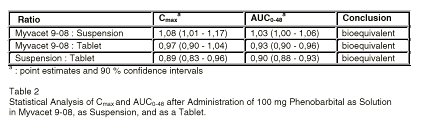 Table 2
Table 2
The results of the statistical analysis of Tmax of phenobarbital from the doasge froms are shown in Table 3.
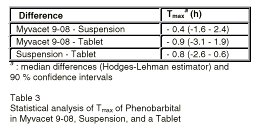 Table 3
Table 3
Discussion and Conclusion
Previous pharmacokinetic studies in healthy volunteers showed, that phenobarbital administered as a tablet has almost complete bioavailability. Literature is sparse on the pharmacokinetics after oral administration of a suspension. Our study has demonstrated, that the suspension and the tablet are bioequivalent. The individual data show, that in most subjects the absorption of phenobarbital from the suspension is faster than from the tablet with a smaller Cmax and AUC0-48. Besides the disadvantages of using a suspension, for patients who find it difficult to swallow tablets, the formulation of the suspension of phenobartbital as we studied it, could be a good alternative.
For drugs with a low aqueous solubility microemulsions or self-emulsifying drug delivery systems may be considered as a formulation for oral administration. We showed, that phenobarbital dissolved in Myvacet 9-08 and formulated without addition of an emulsifier, has a good oral absorption profile and is bioequivalent to a tablet and the suspension. Myvacet 9-08 might prove to be an excellent vehicle to enhance the intestinal absorption of a co-formulated drug, therefore it should be studied with other drugs with a known bioavailability problem.
References
1. Constantinides P.P.
Lipid microemulsions for improving drug dissolution and oral absorption: physical andbiopharmaceutical aspects. Pharm. Res. 1995; 12: 1561-1572.
2. Shah N.H., Carvajal M.T., Patel C.I., Infeld M.H., Malick A.W. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J.Pharm. 1994; 106: 7-14.
3. Acetic and fatty acid esters of glycerol. In: Toxicological evaluation of some food additives. FAO Nutrition meetings report series no. 53A; Rome, 1974: pp 210-213.
4. Mono- and di-acetylated monoglycerides.
The United States Pharmacopeia, USP 23 / The National Formulary, NF 18.
The United States Pharmacopeial Convention Inc.: Rockville MD, 1994: pp 2269-2270.
| Discussion Board | Previous Page | Your Poster Session |